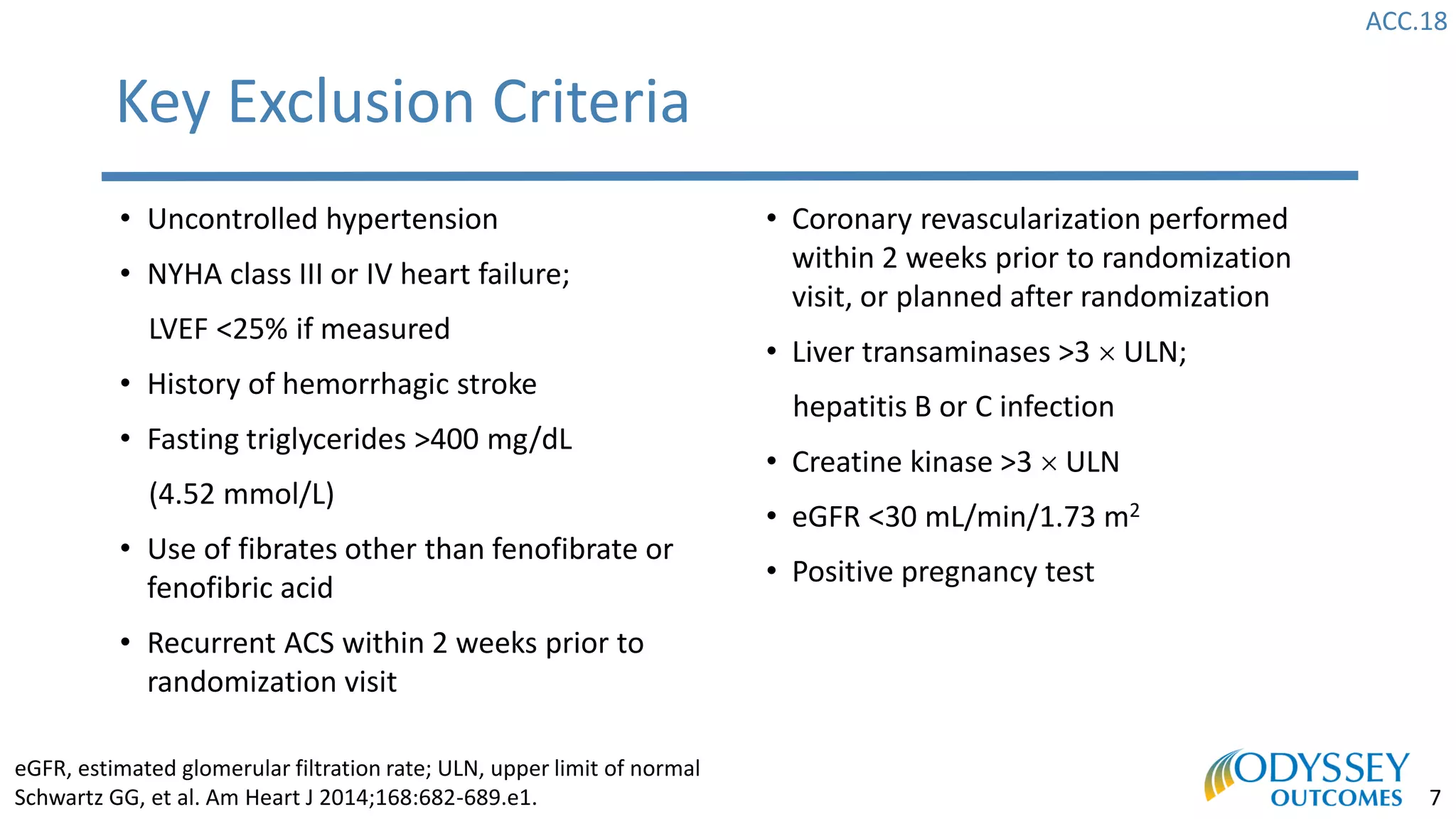
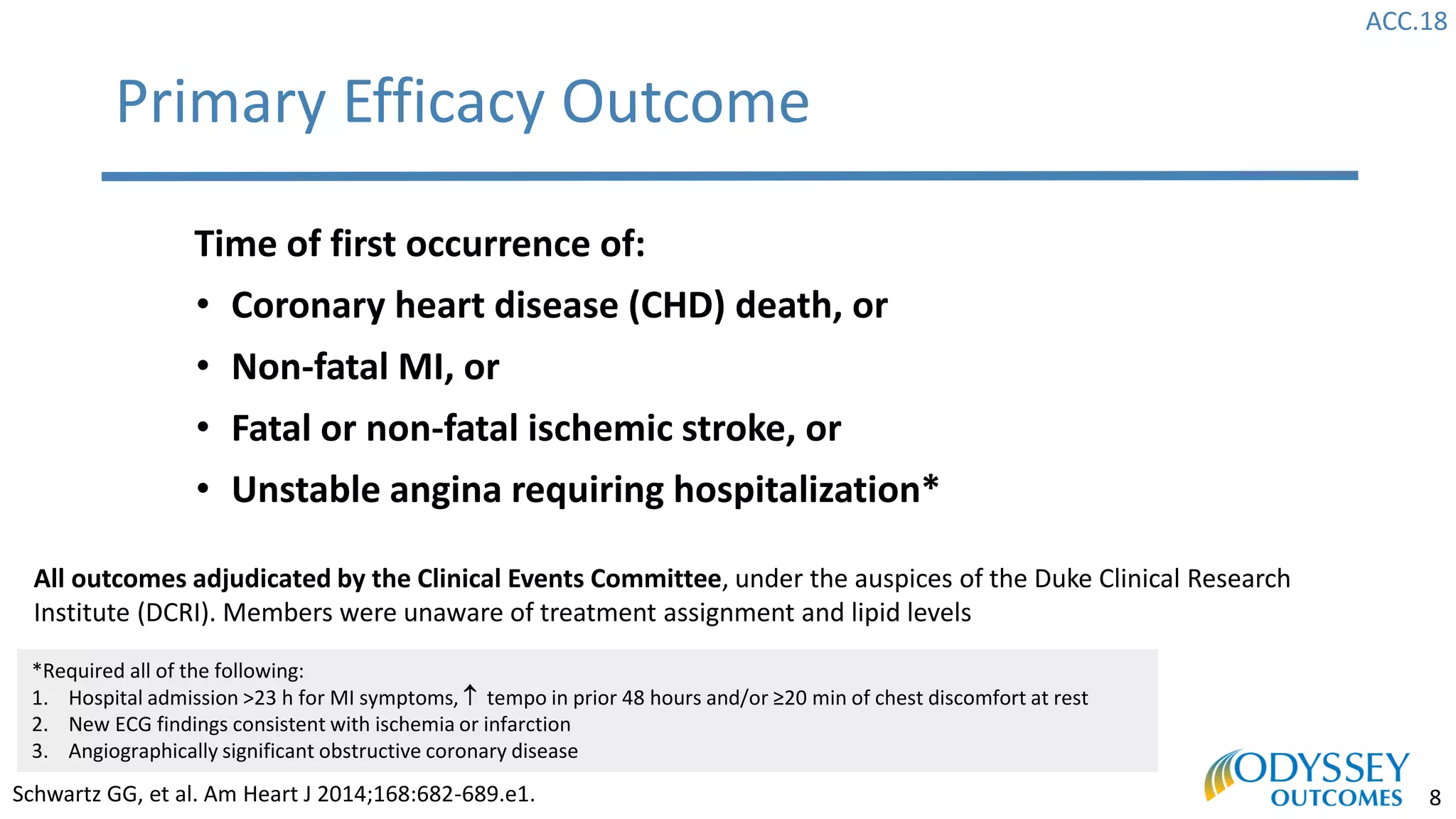
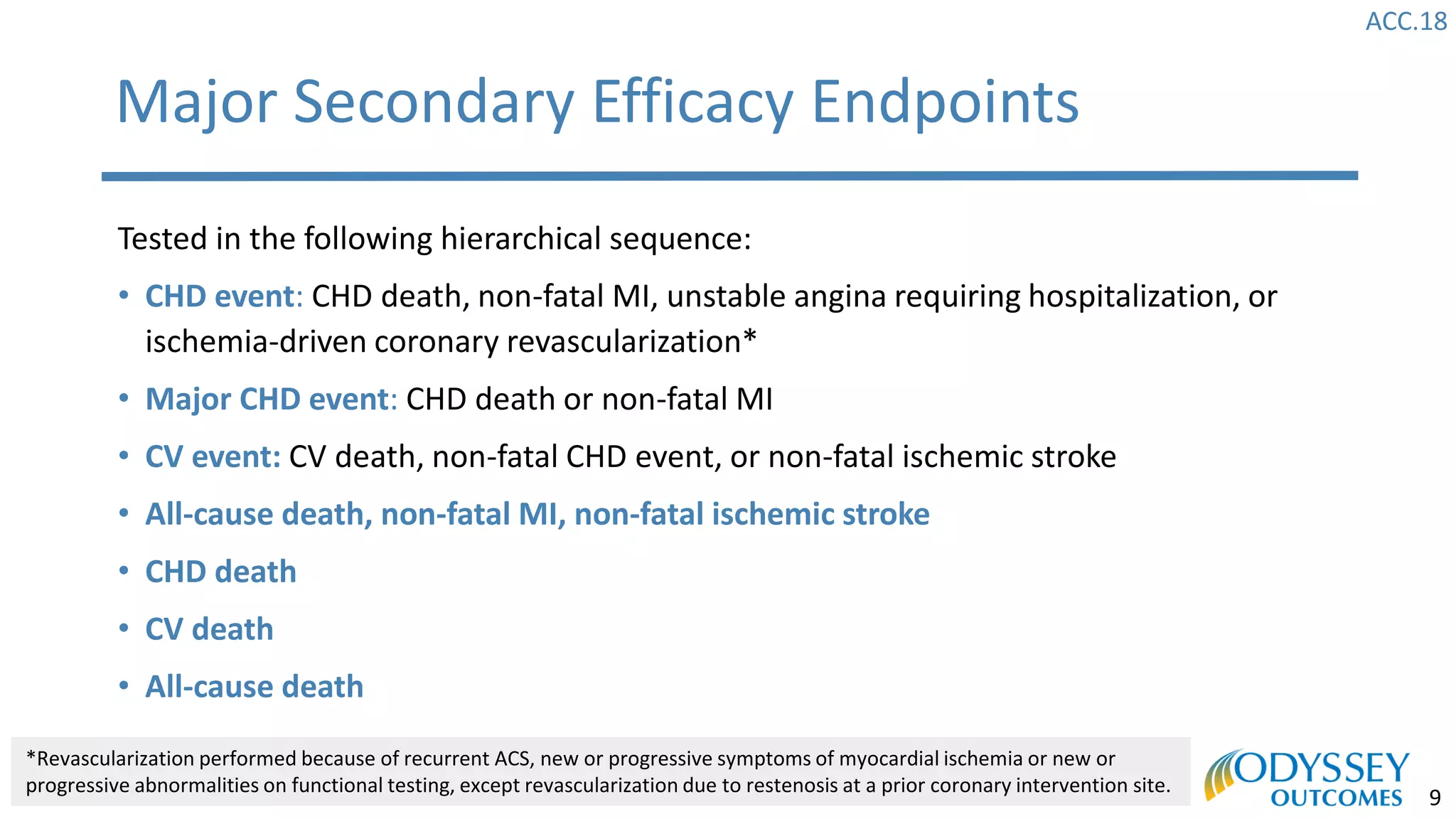
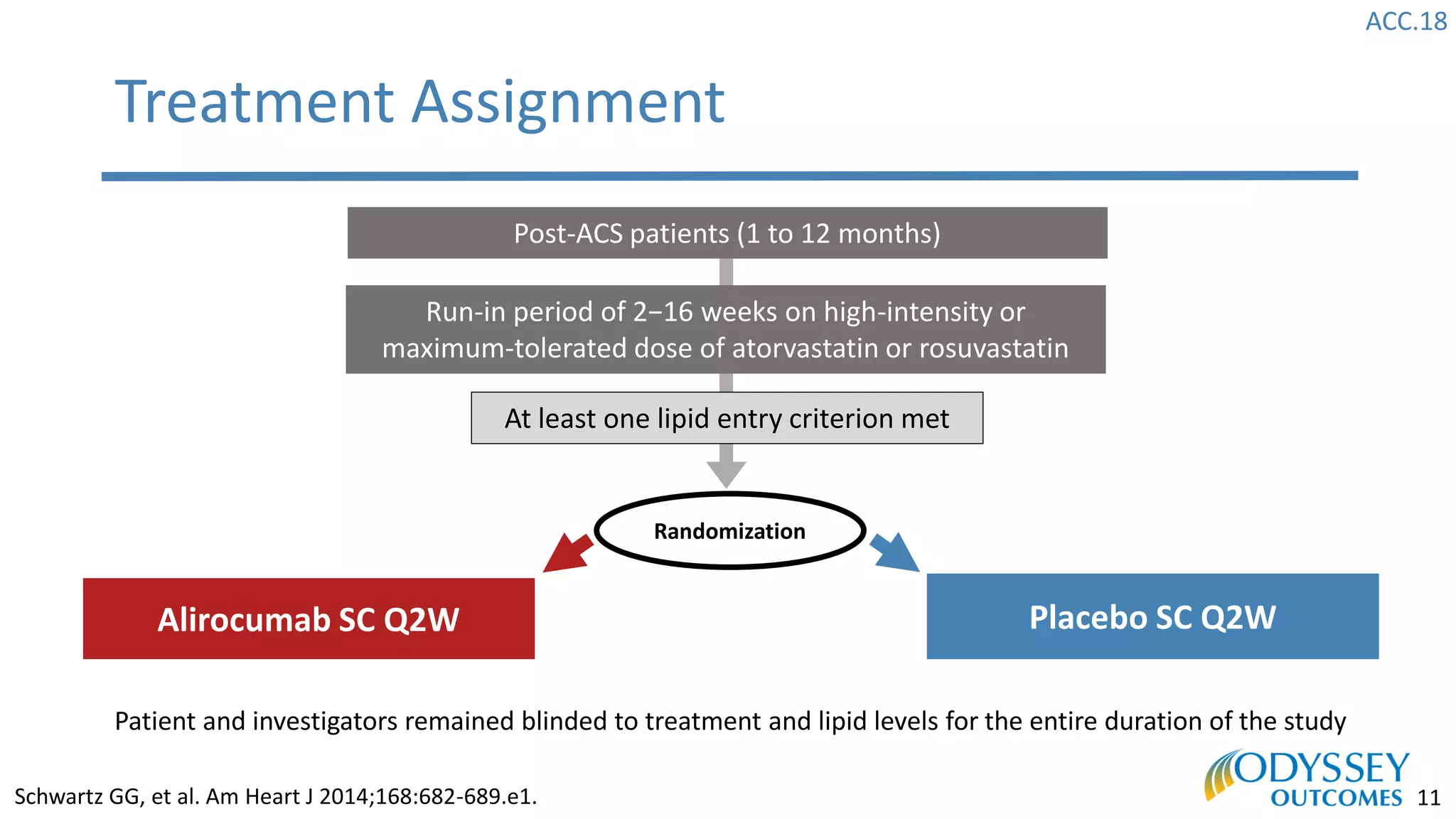
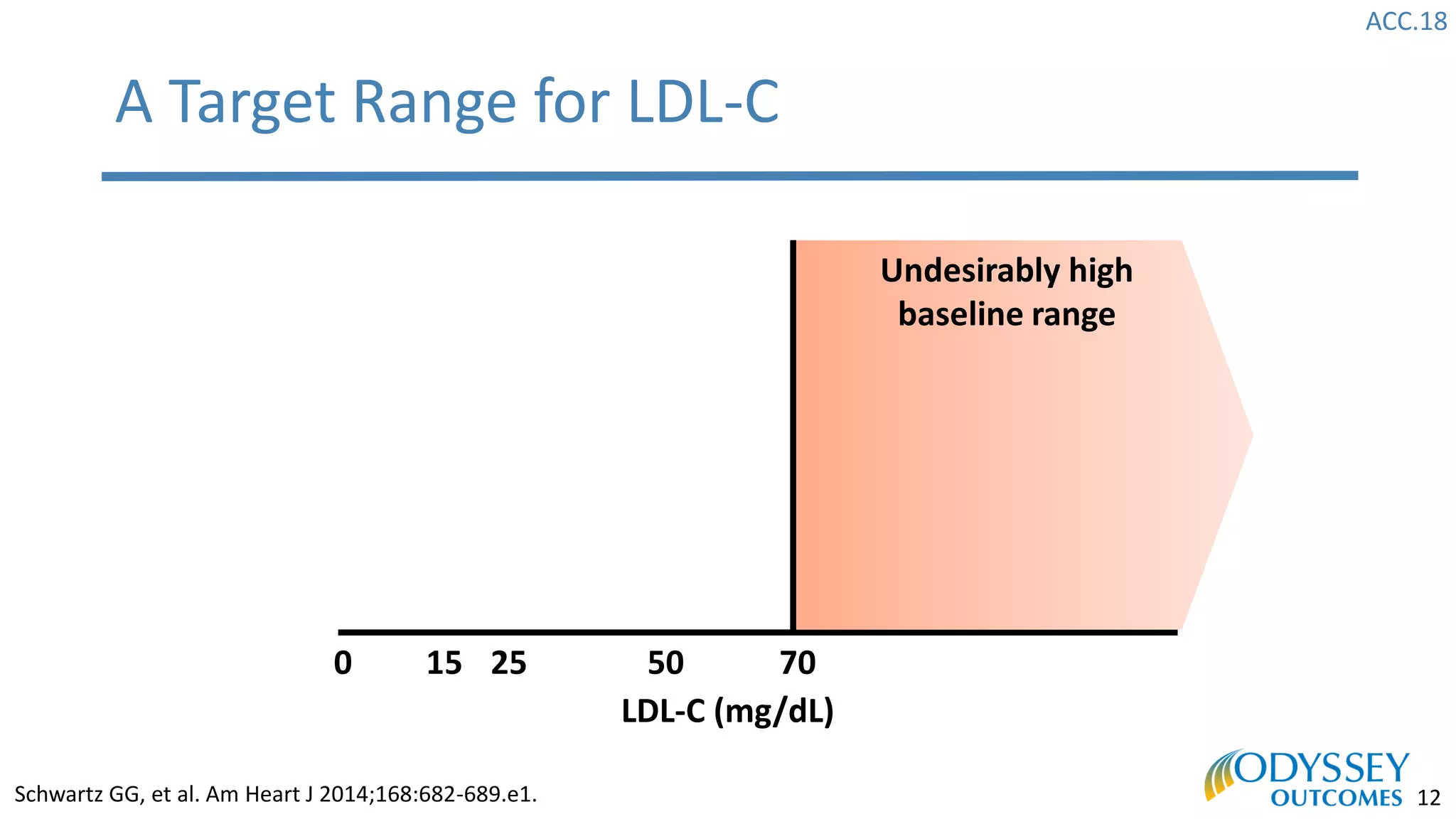
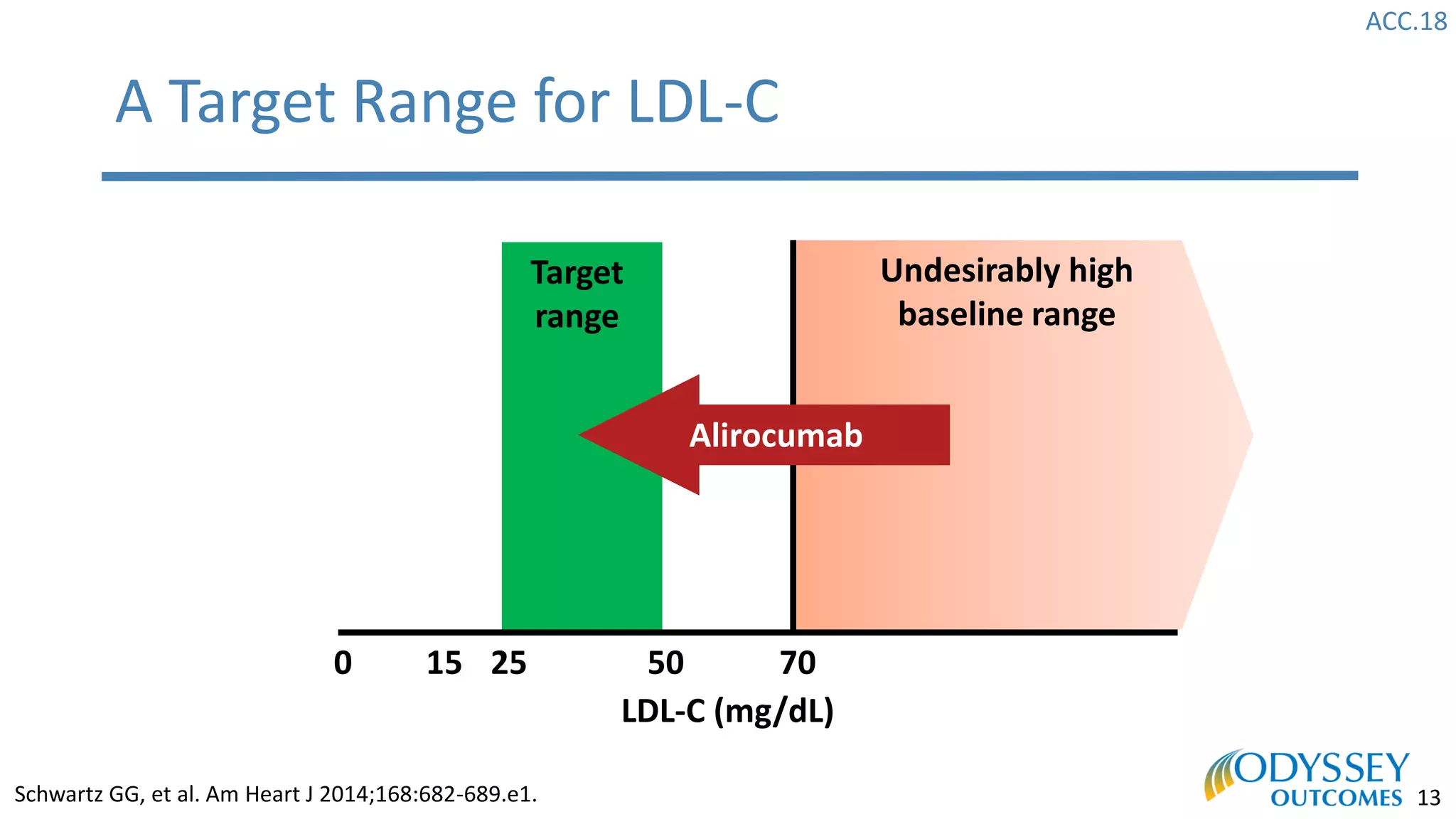
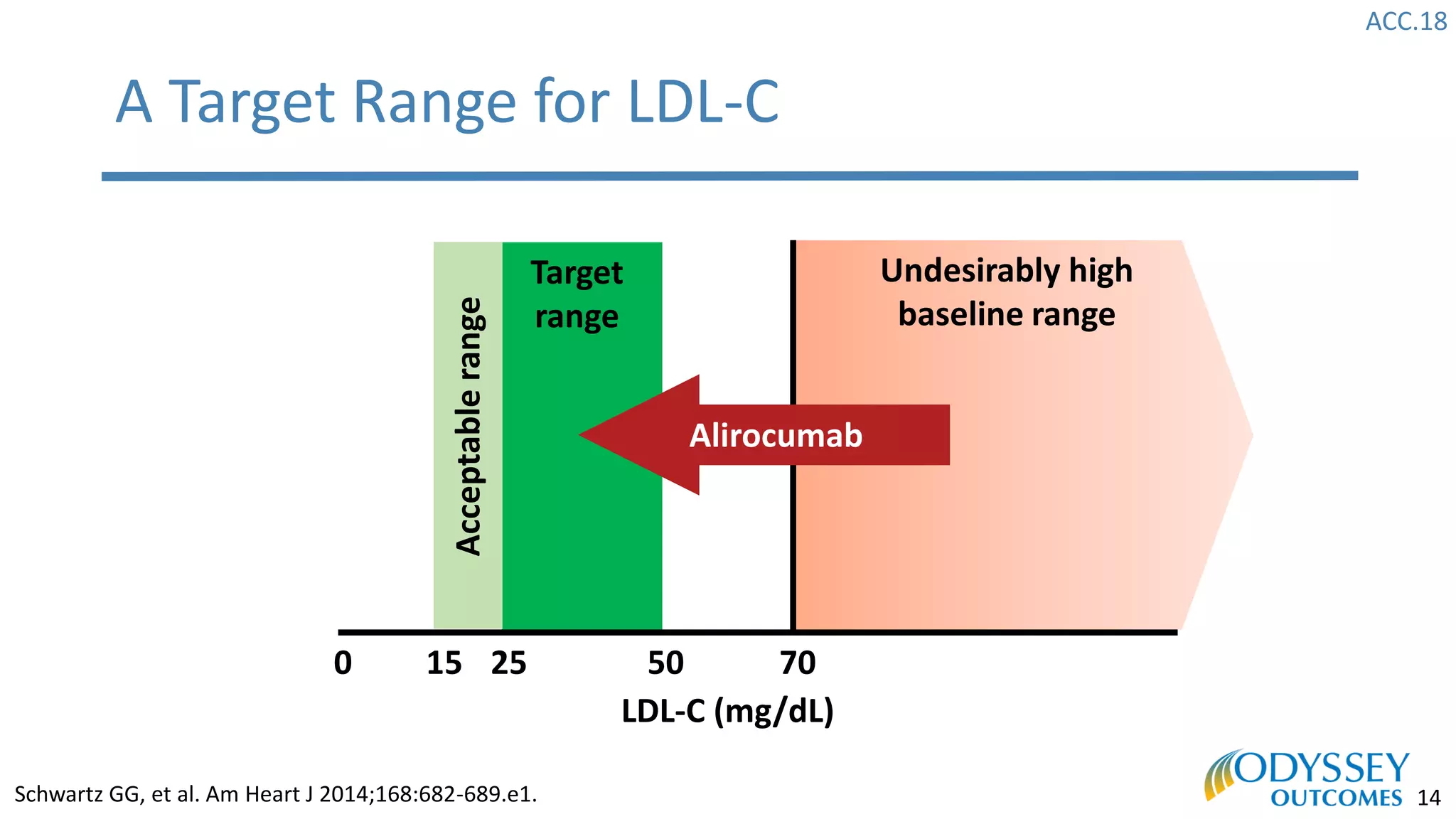
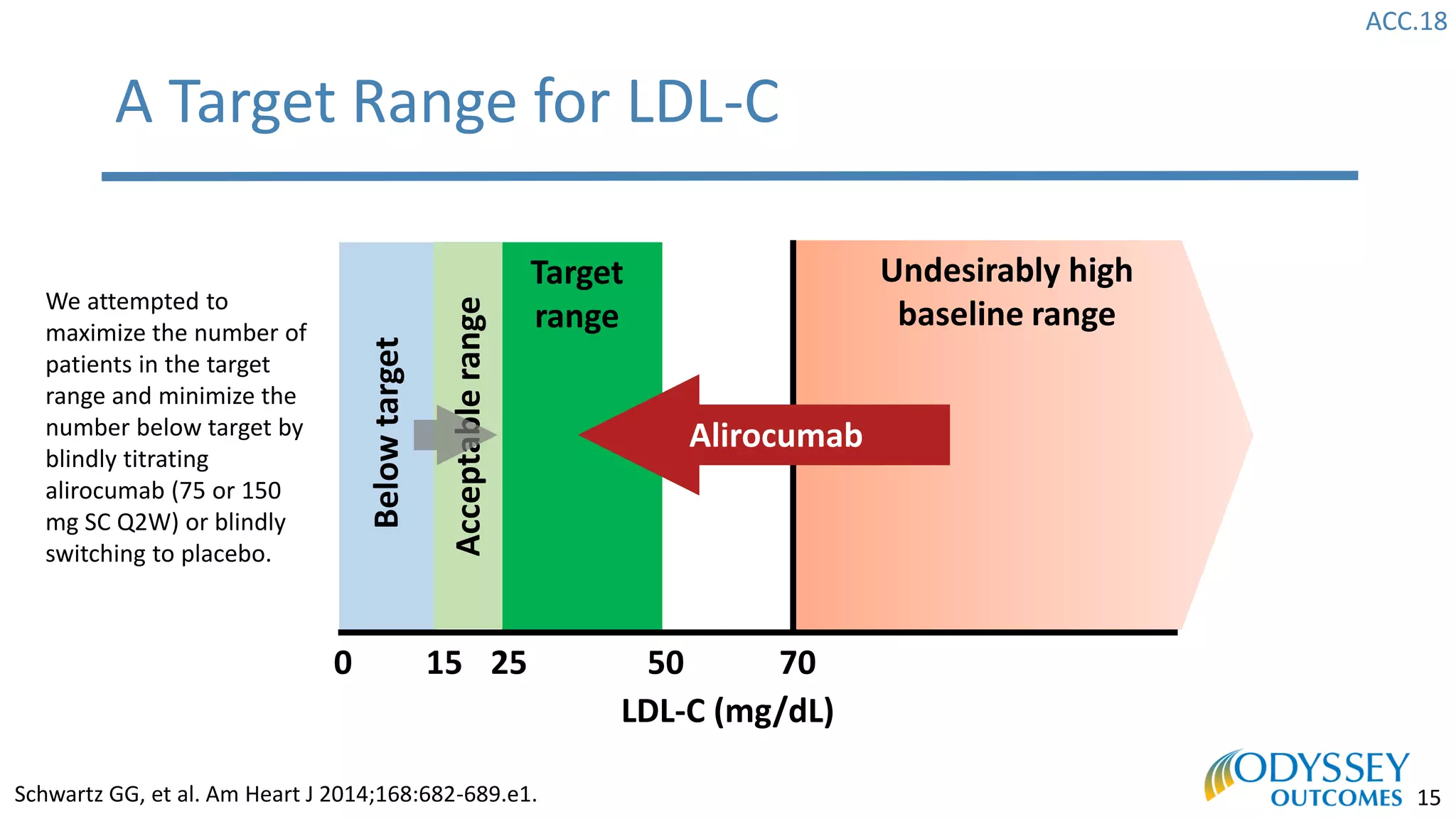
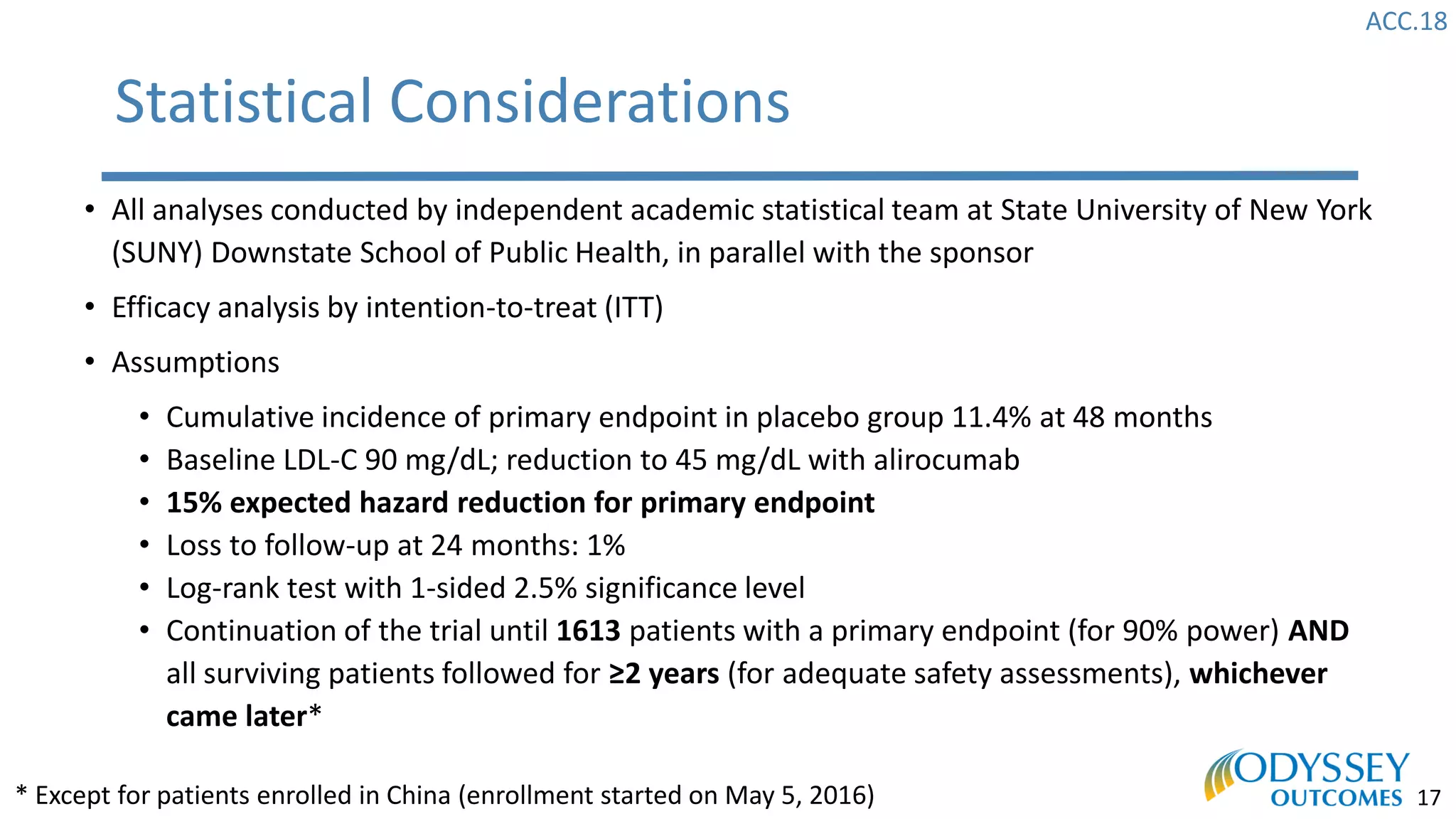
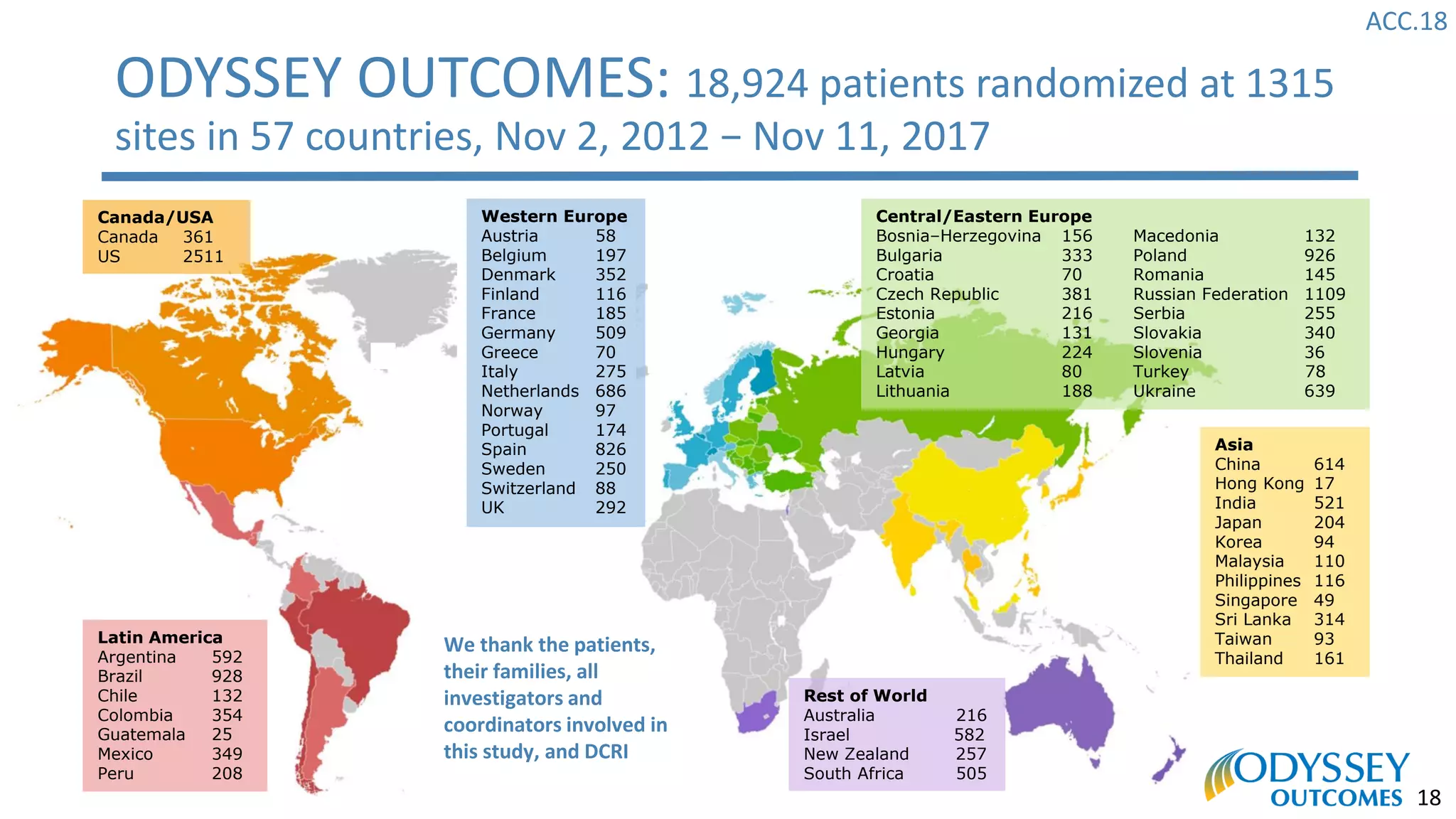
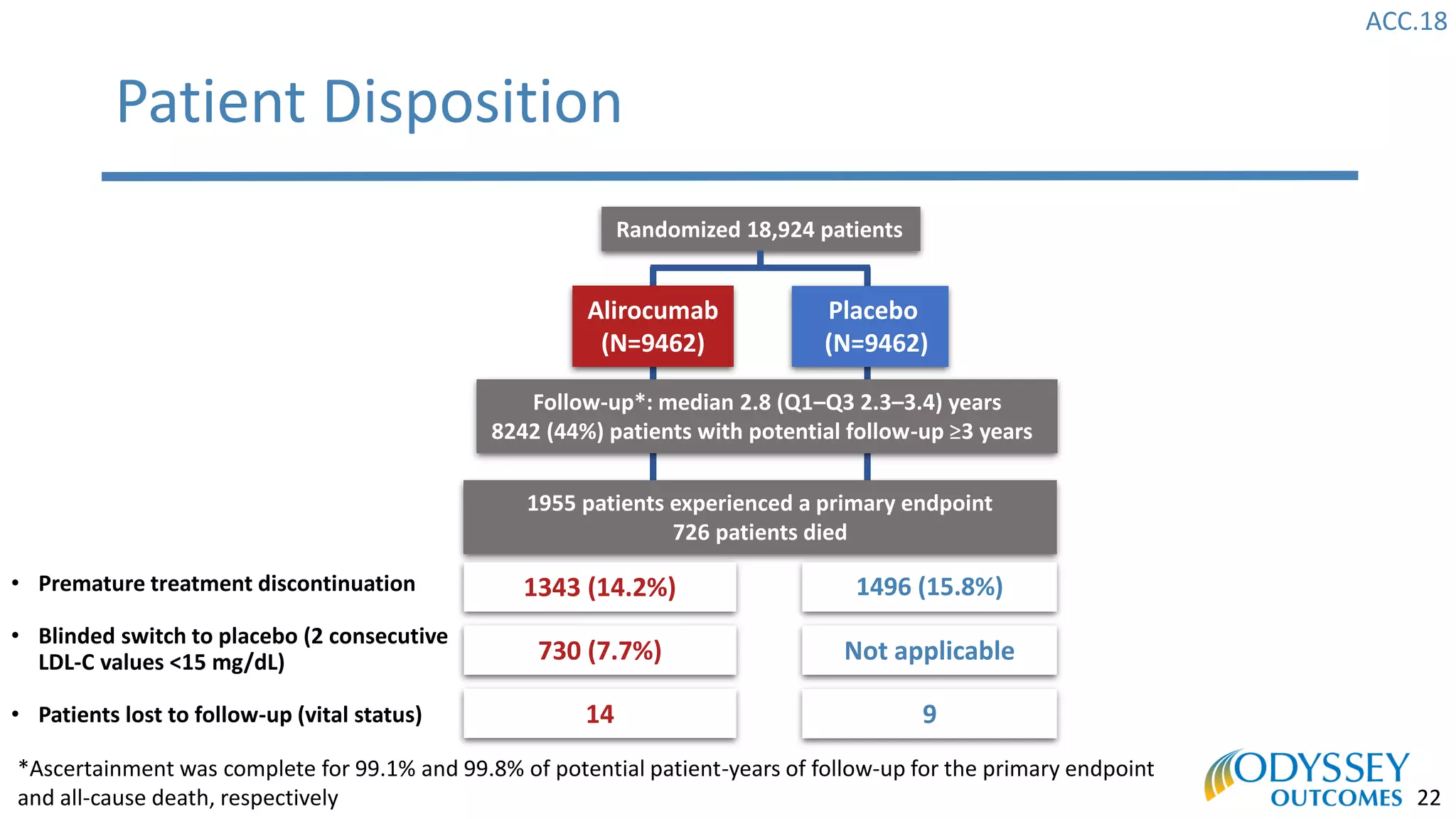
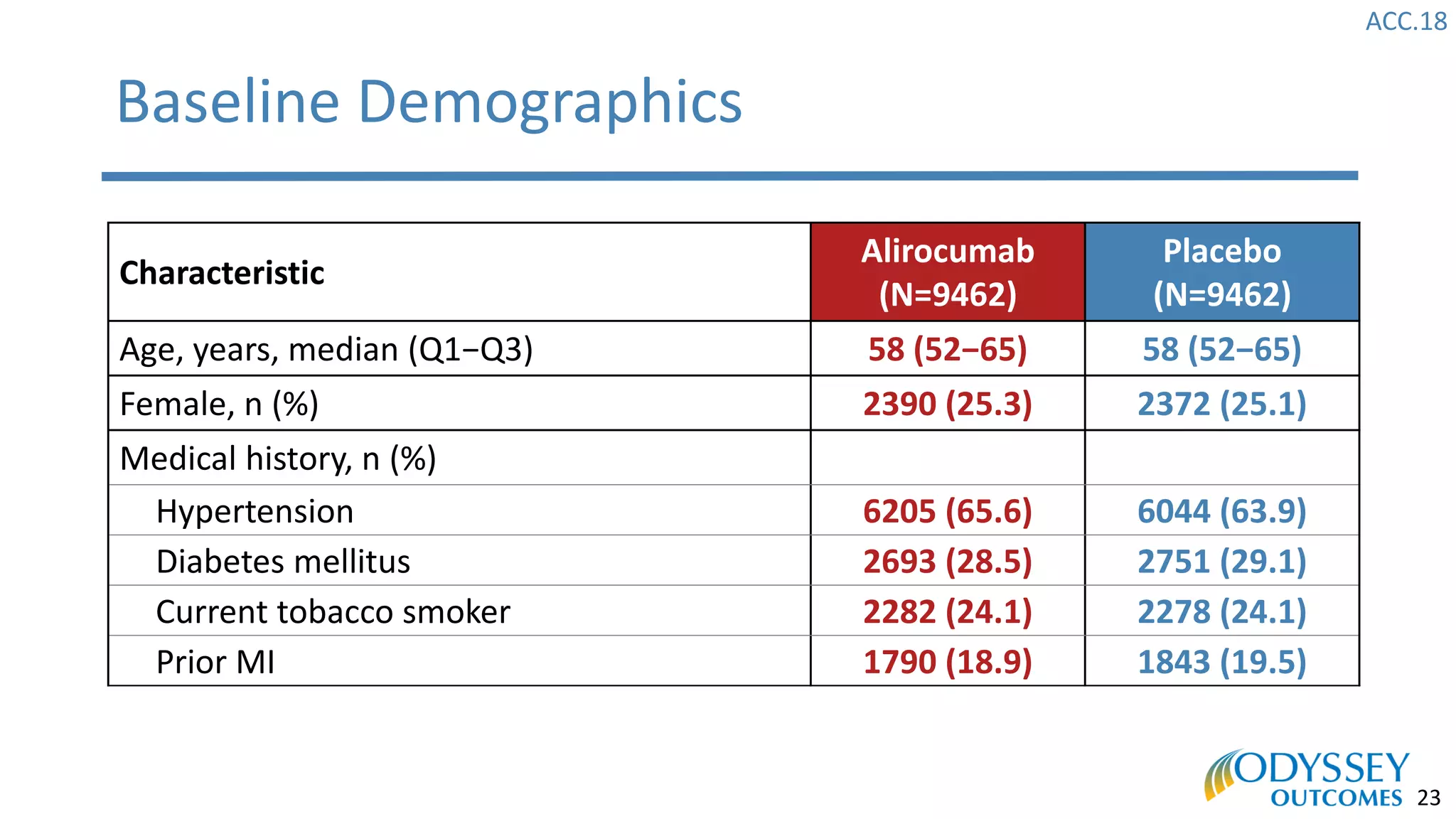
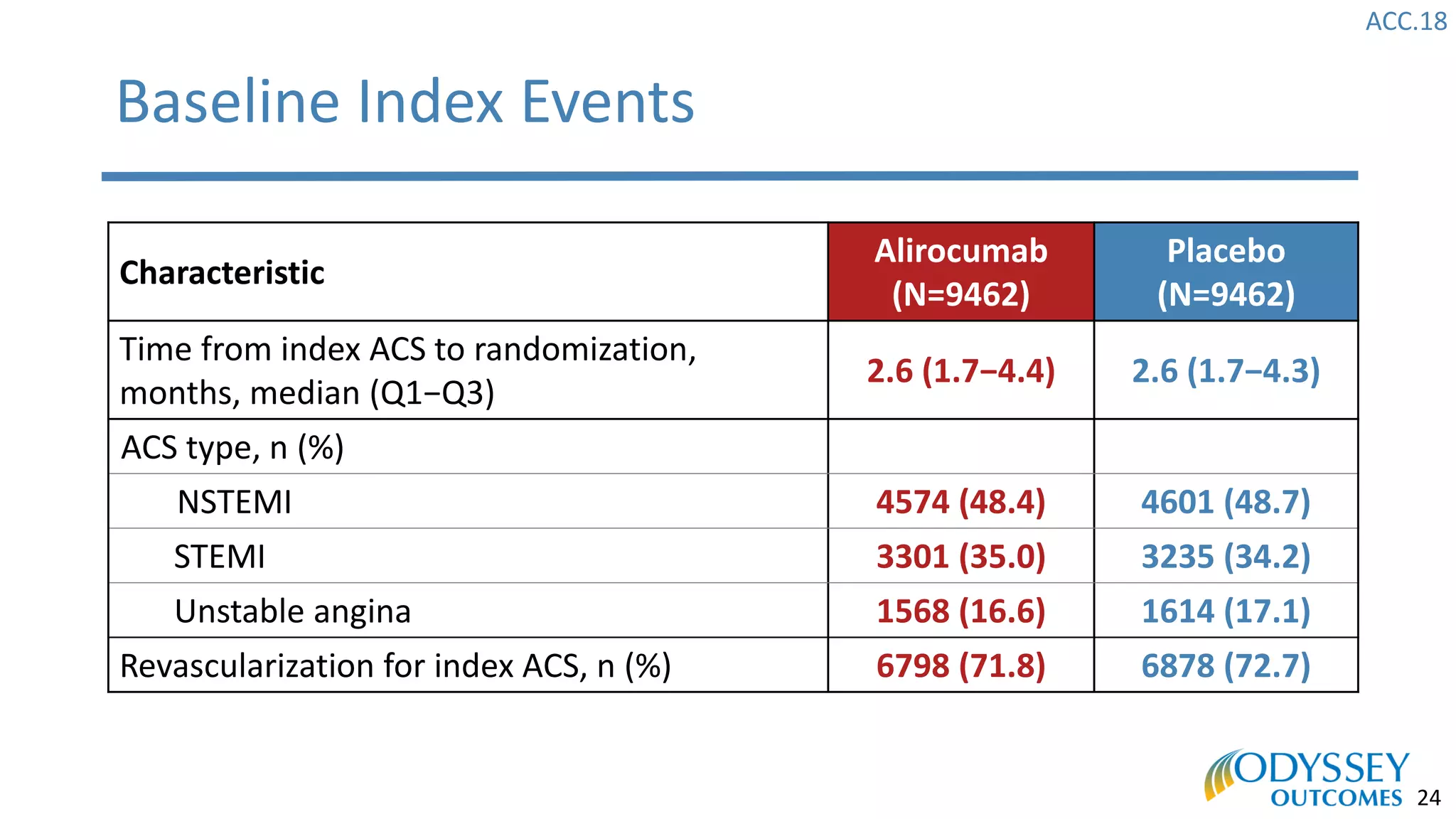
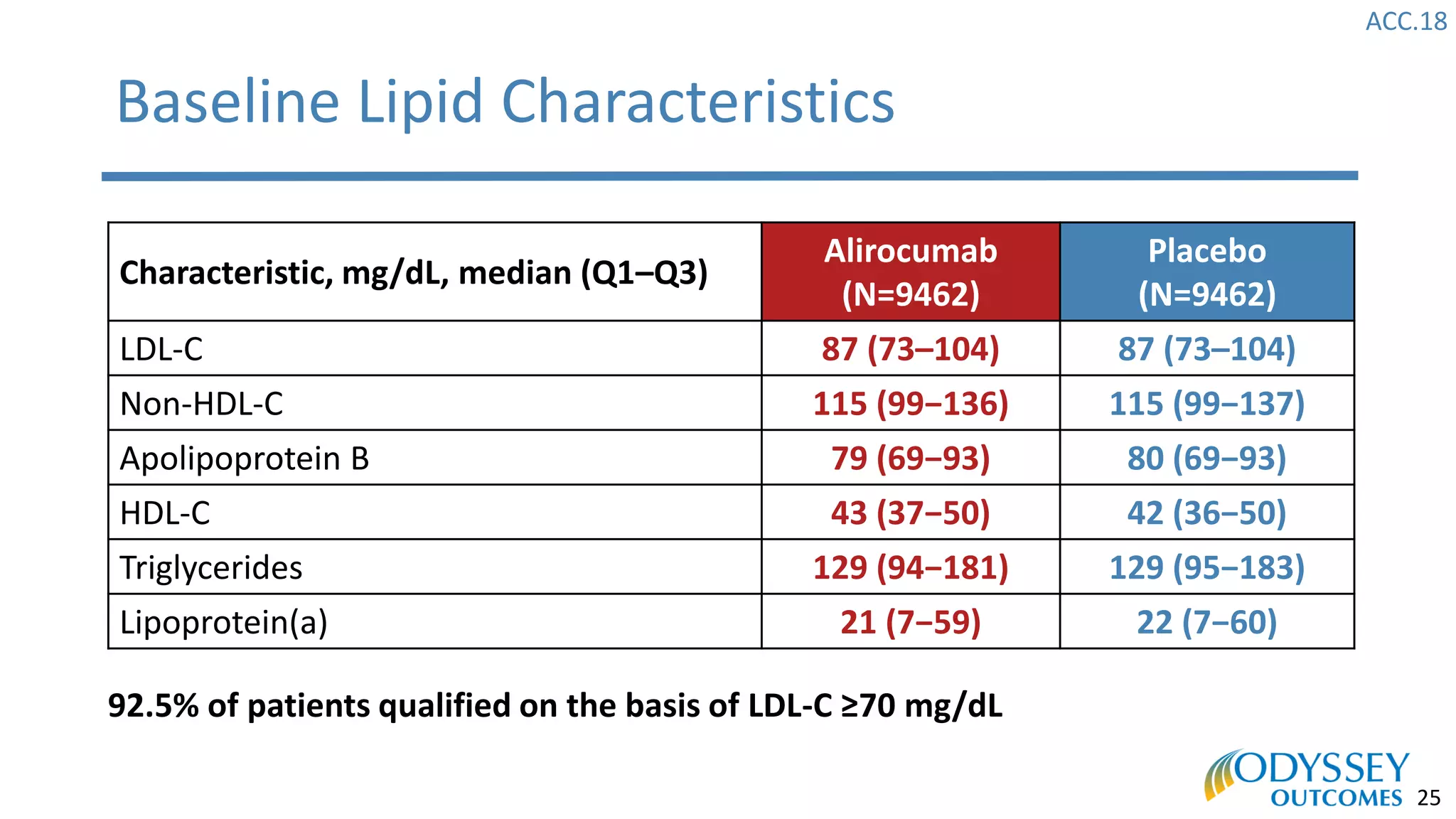
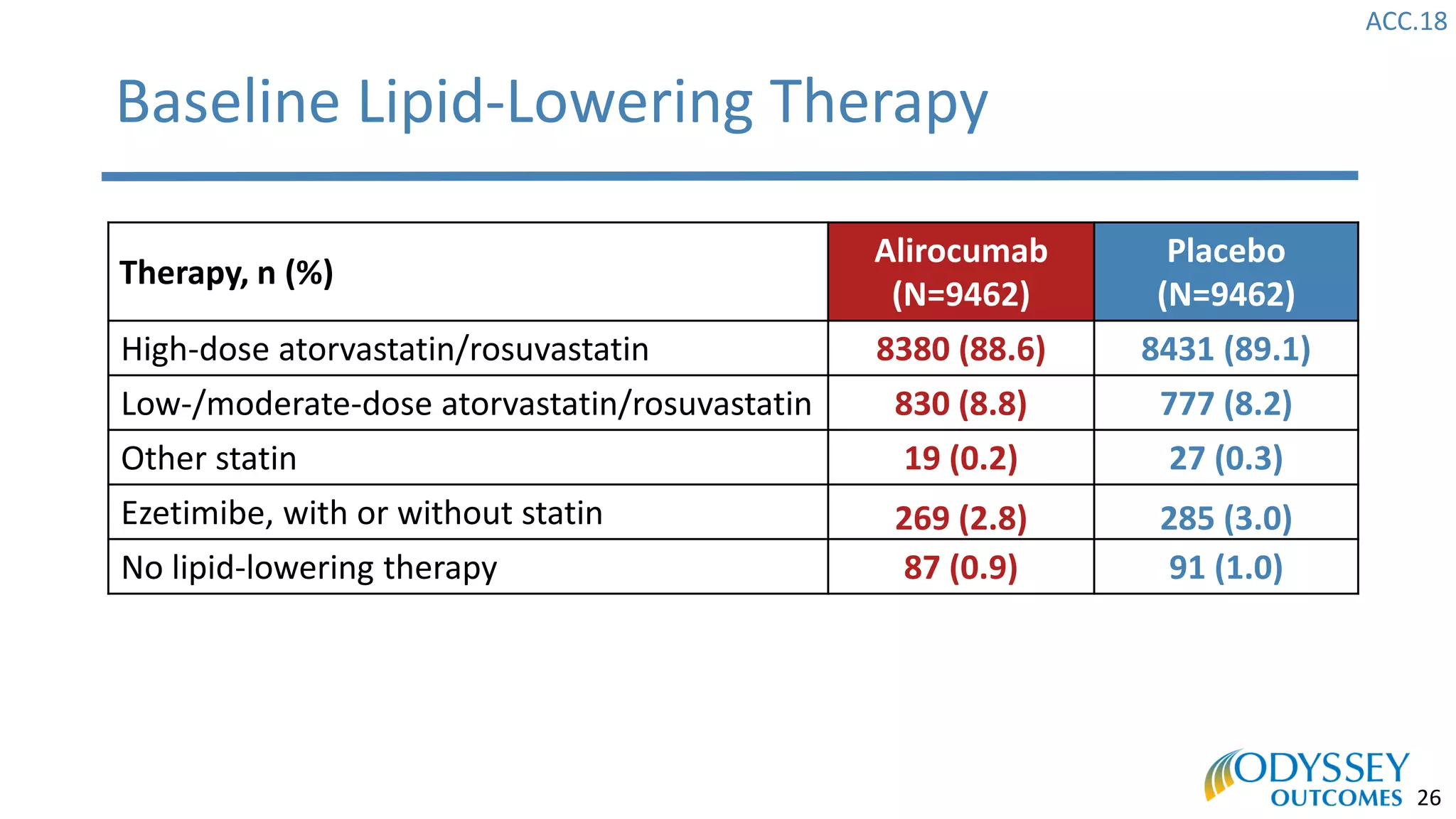
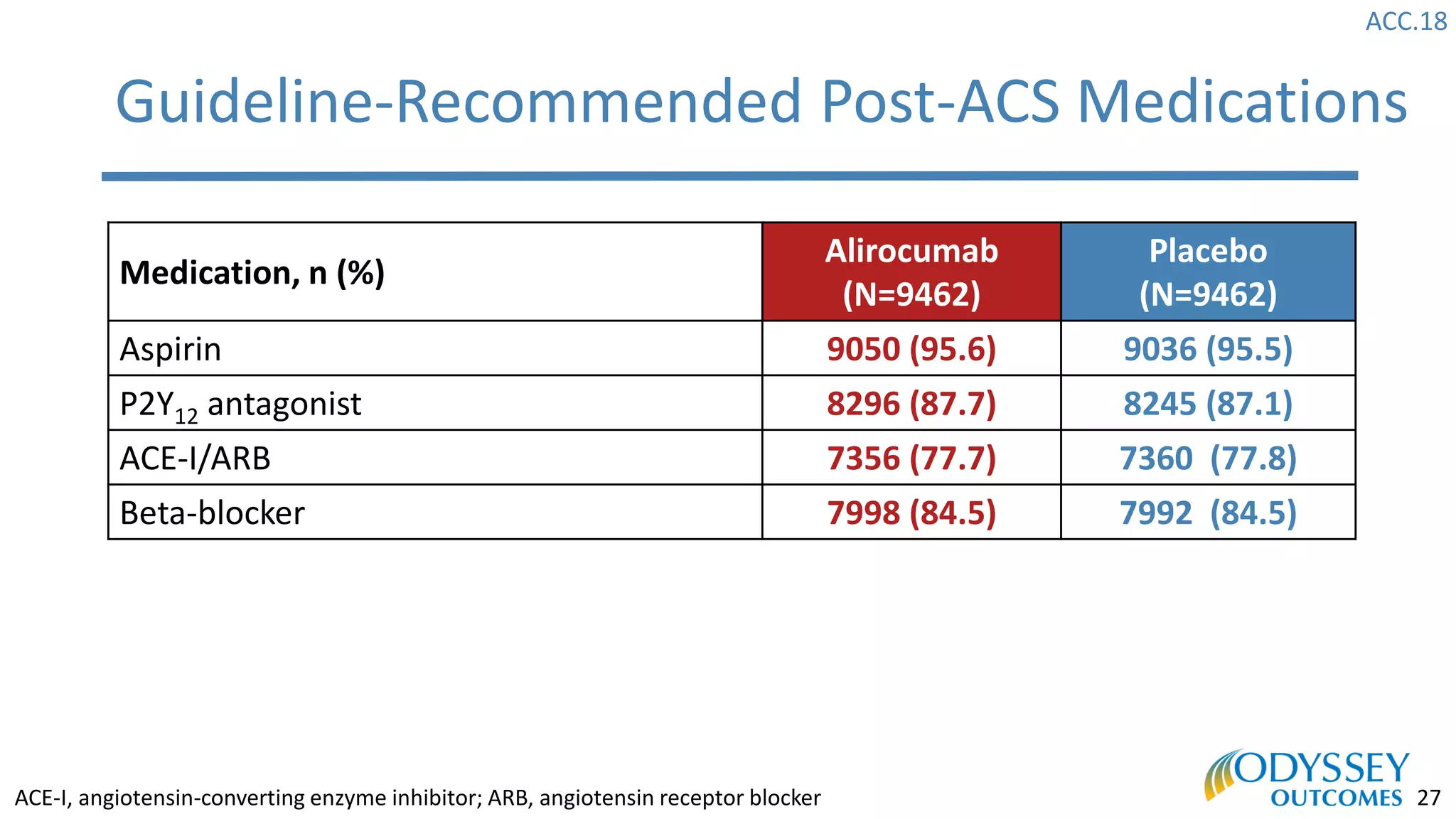
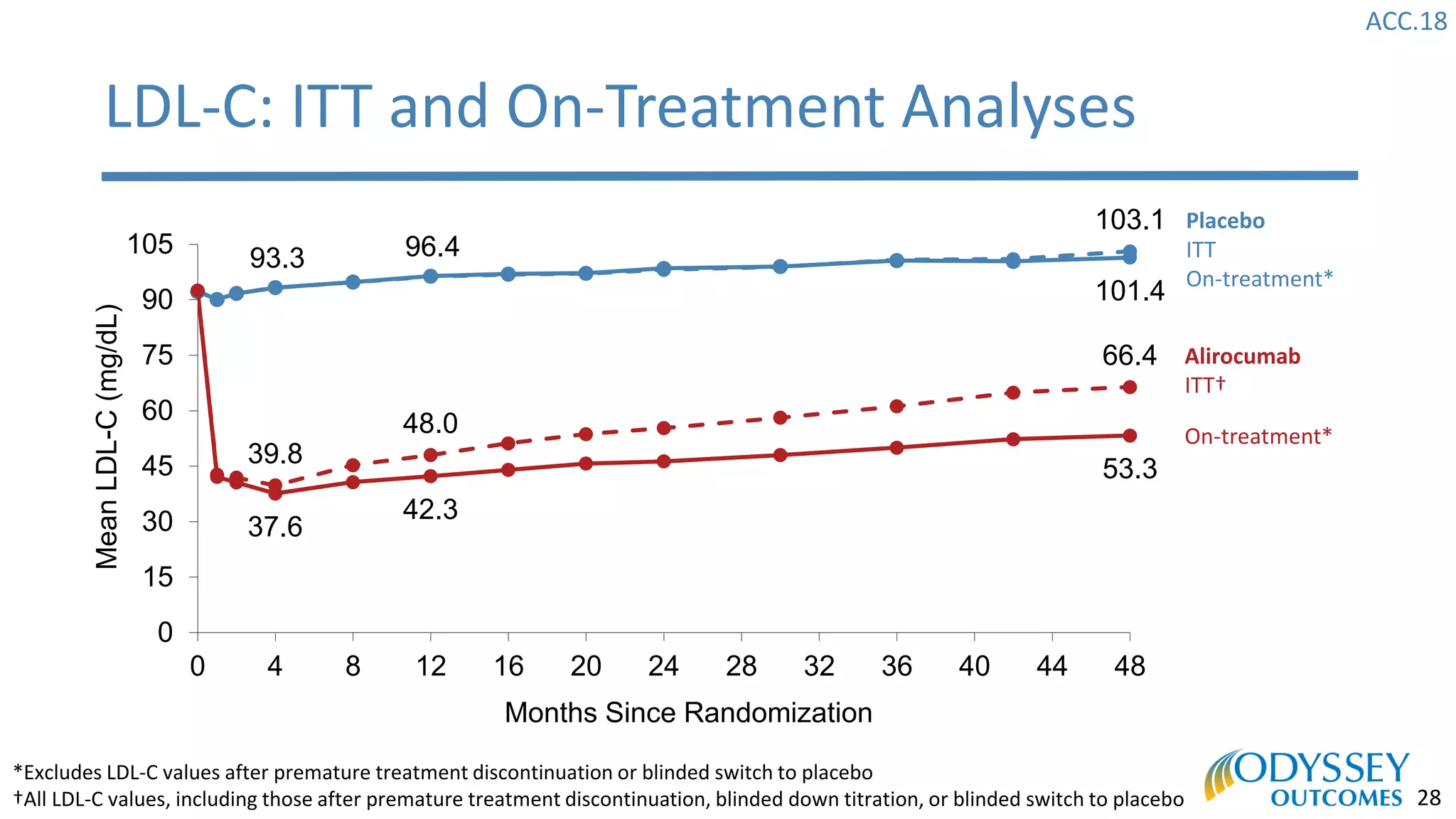
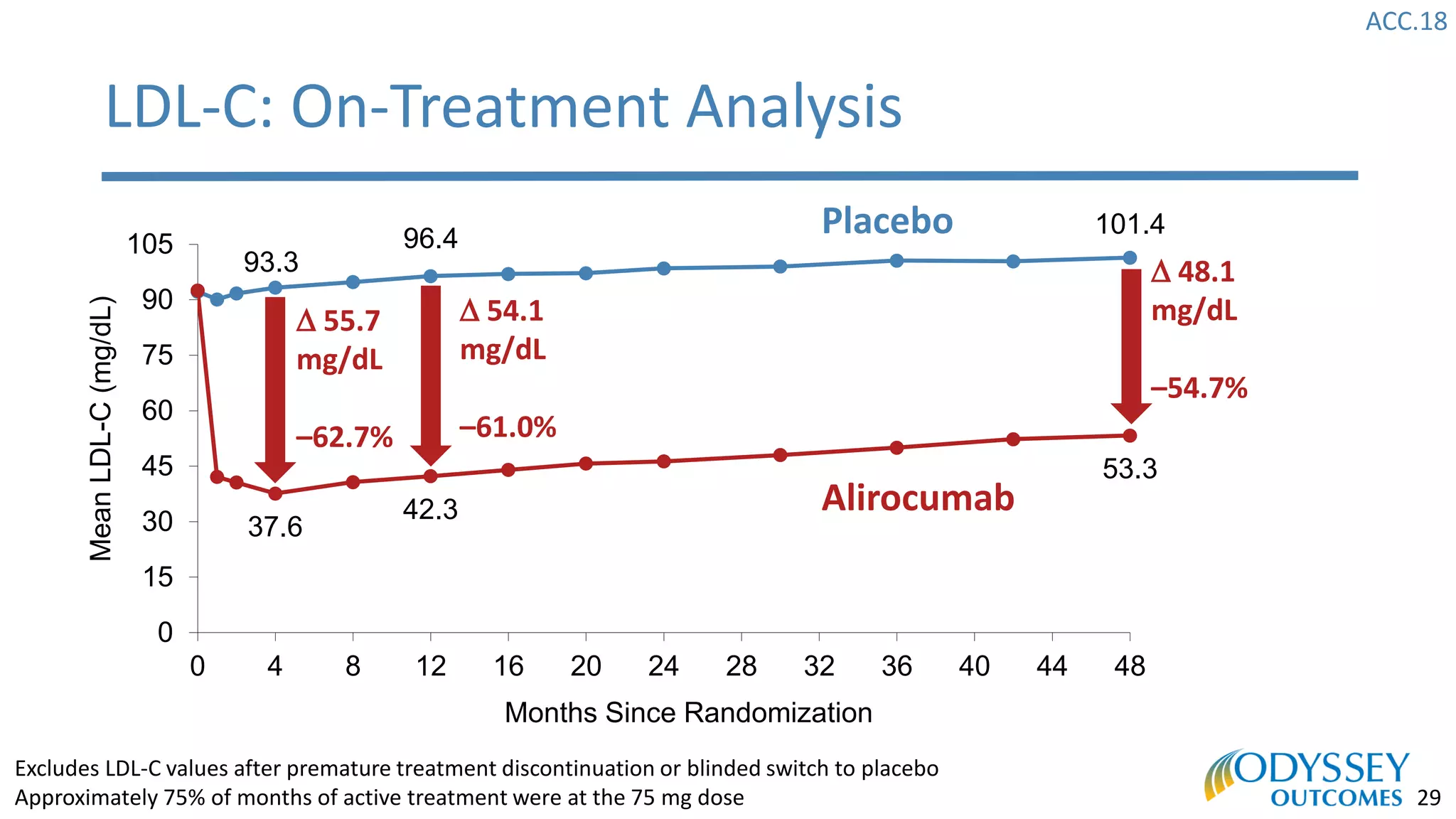
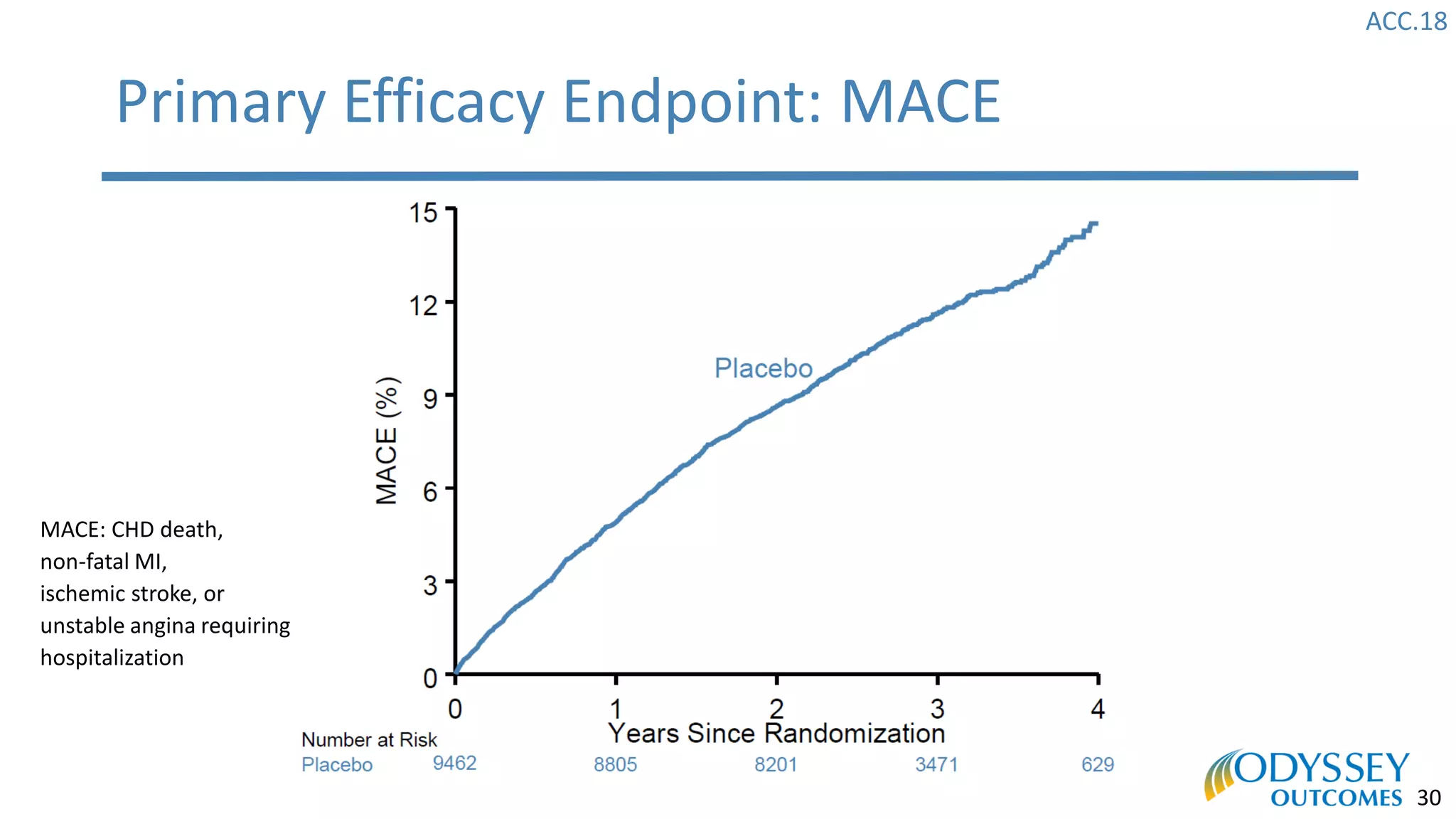
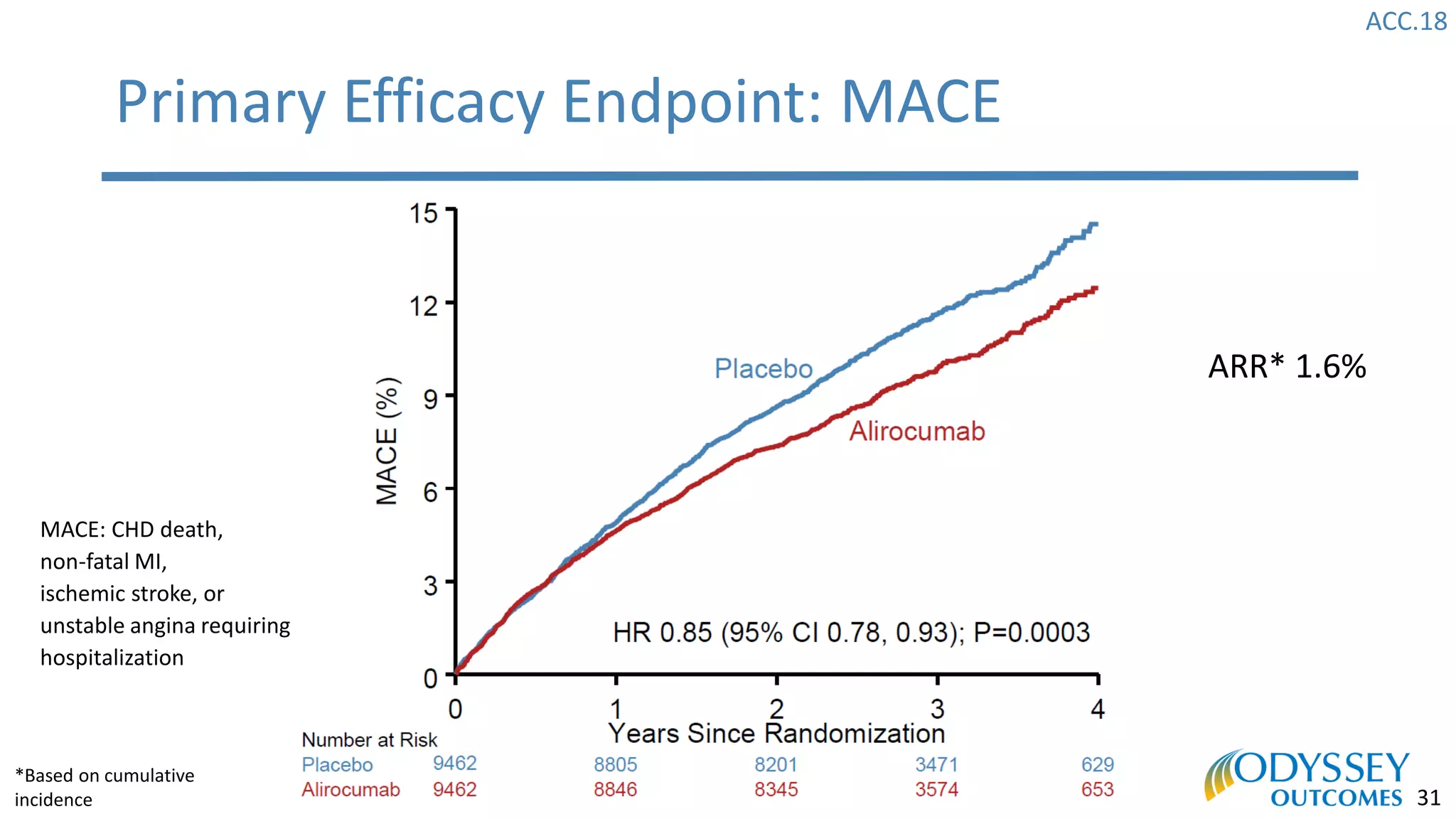
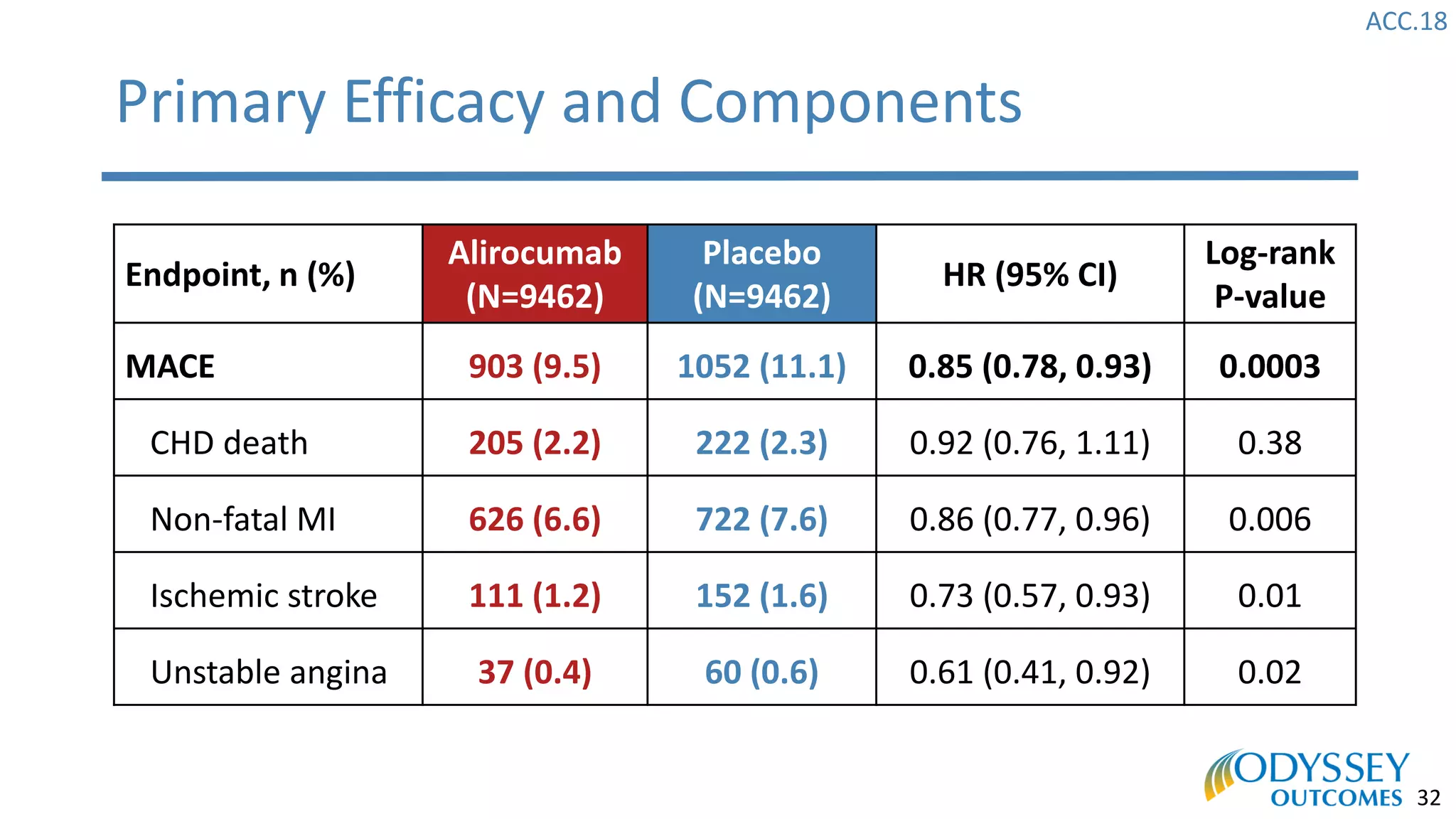
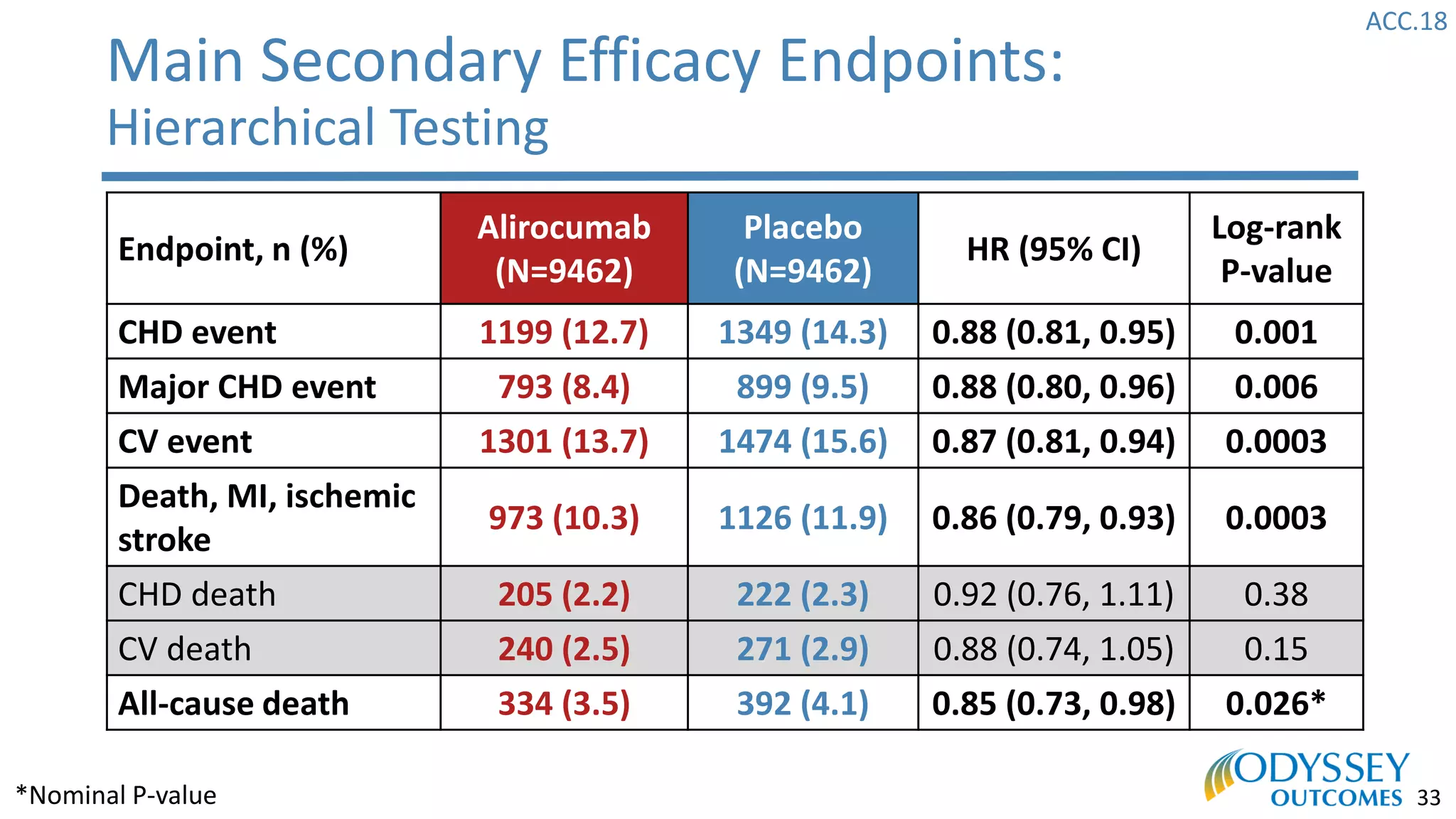
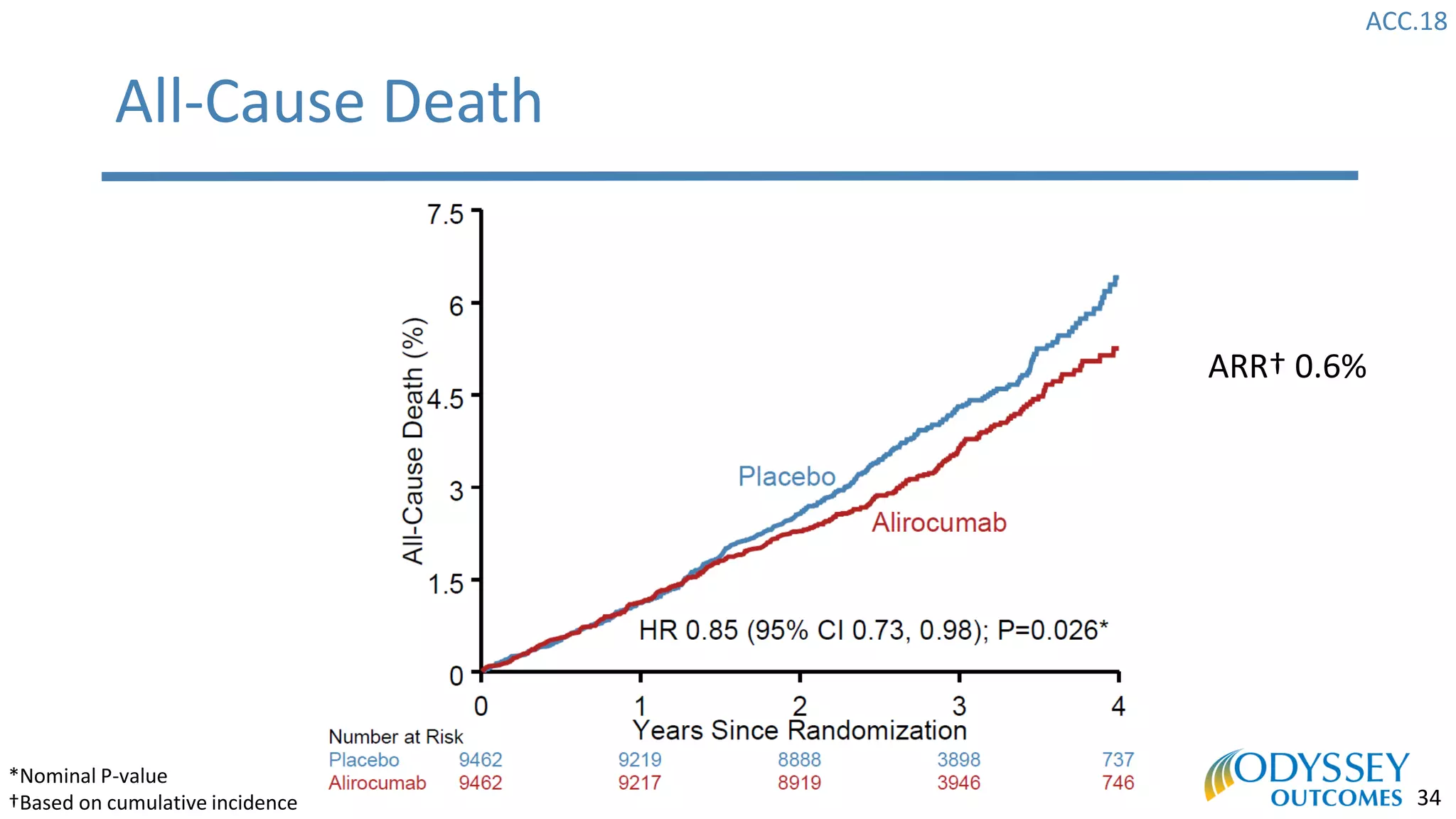
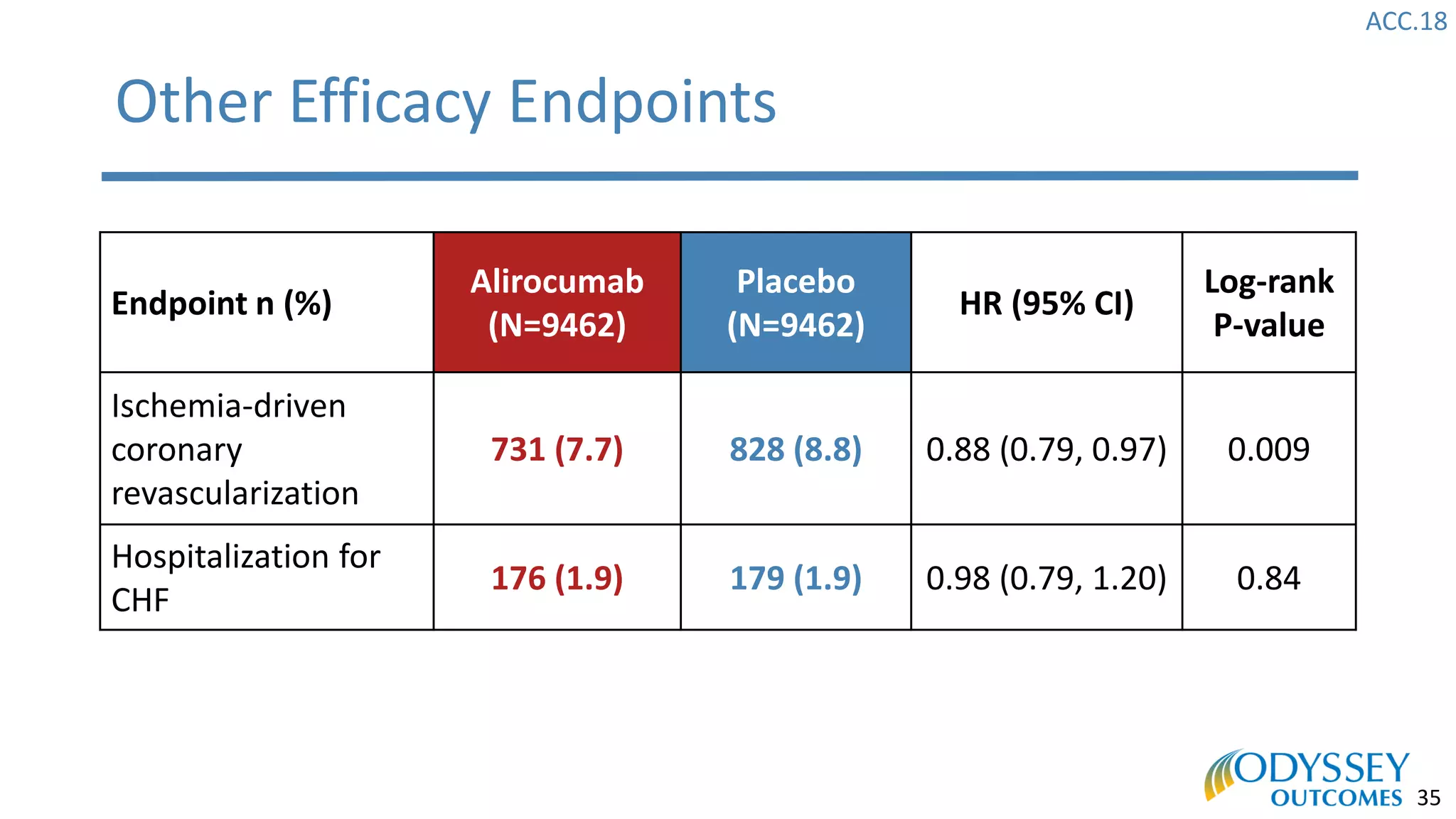
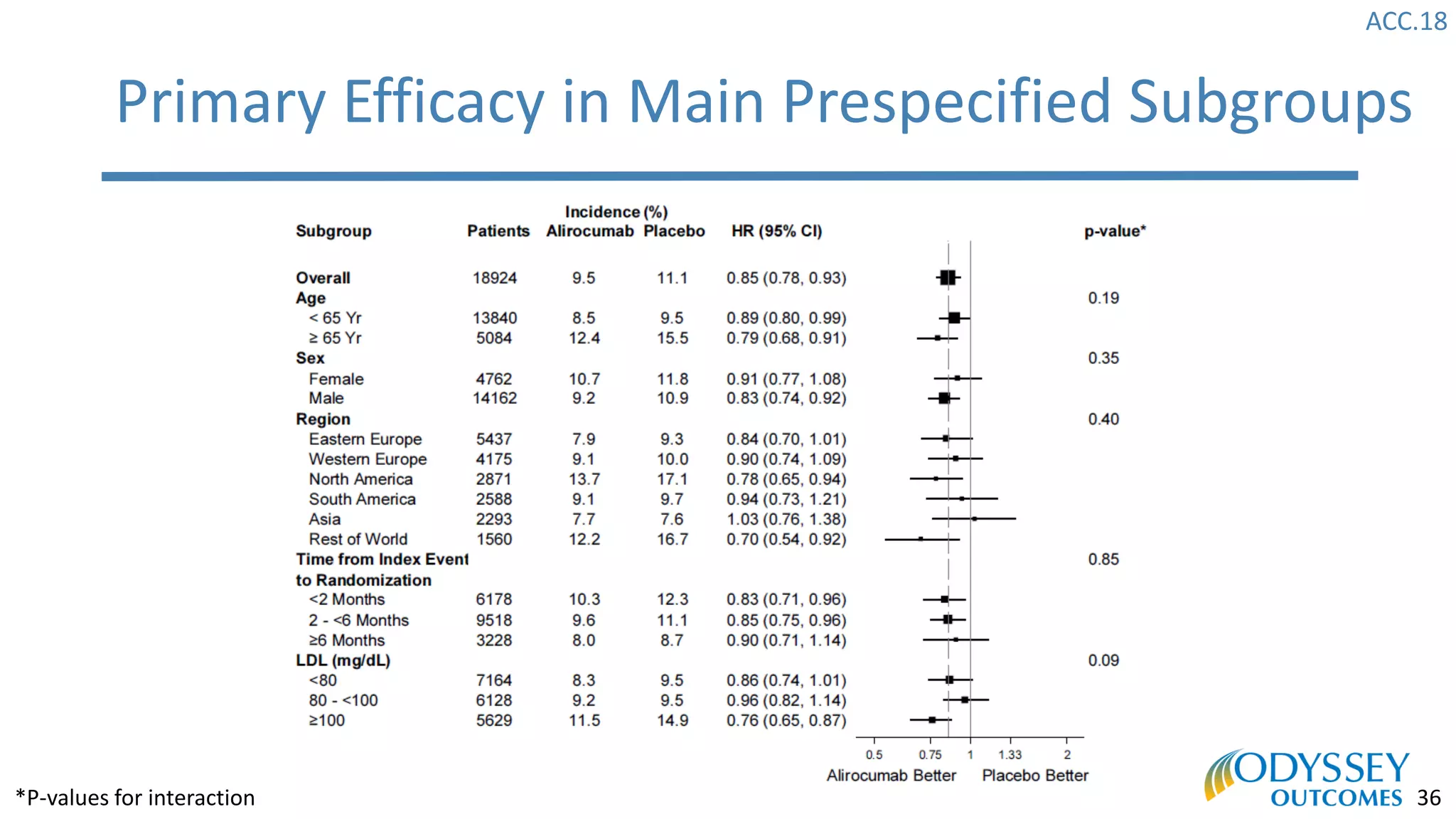
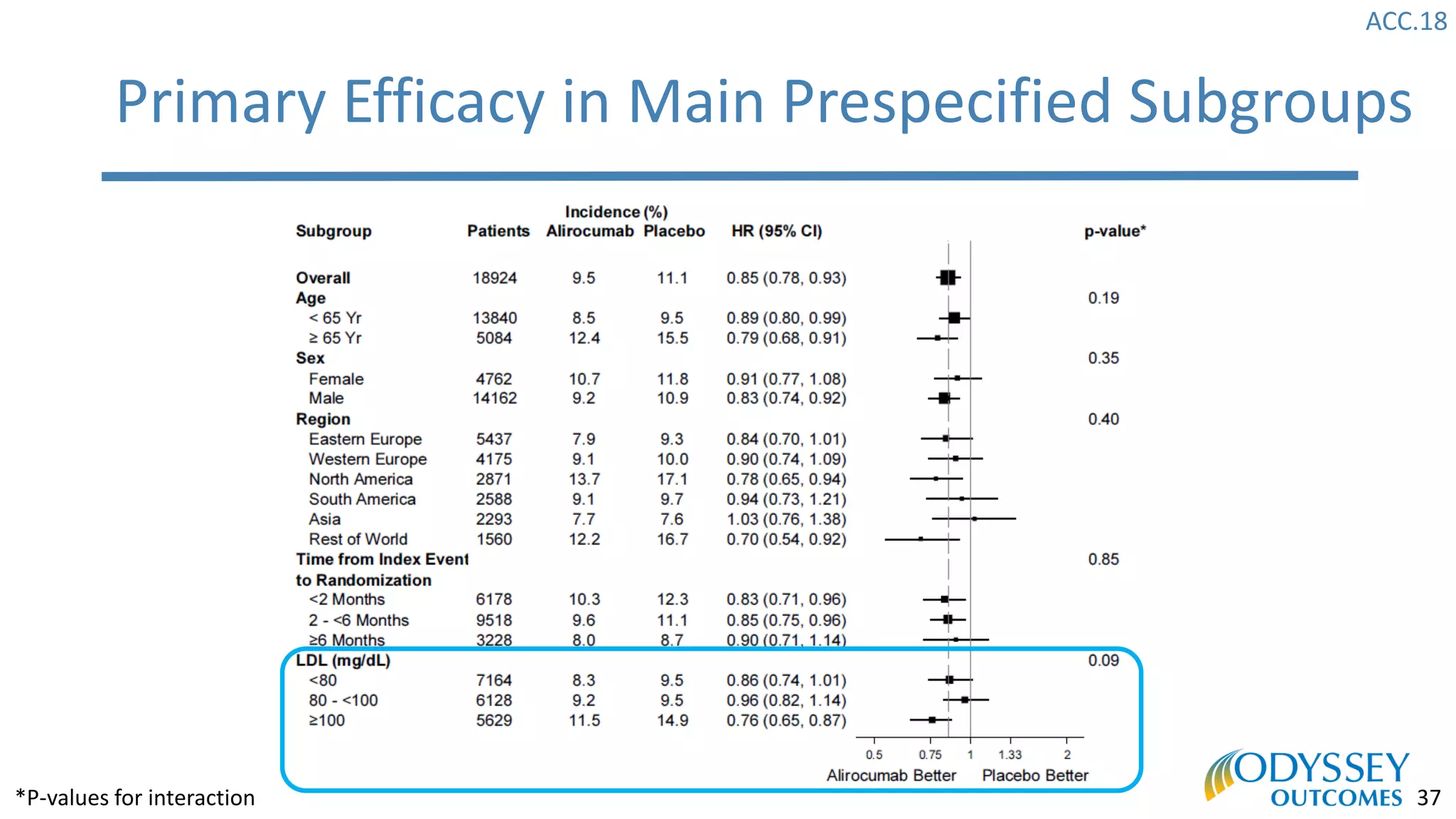
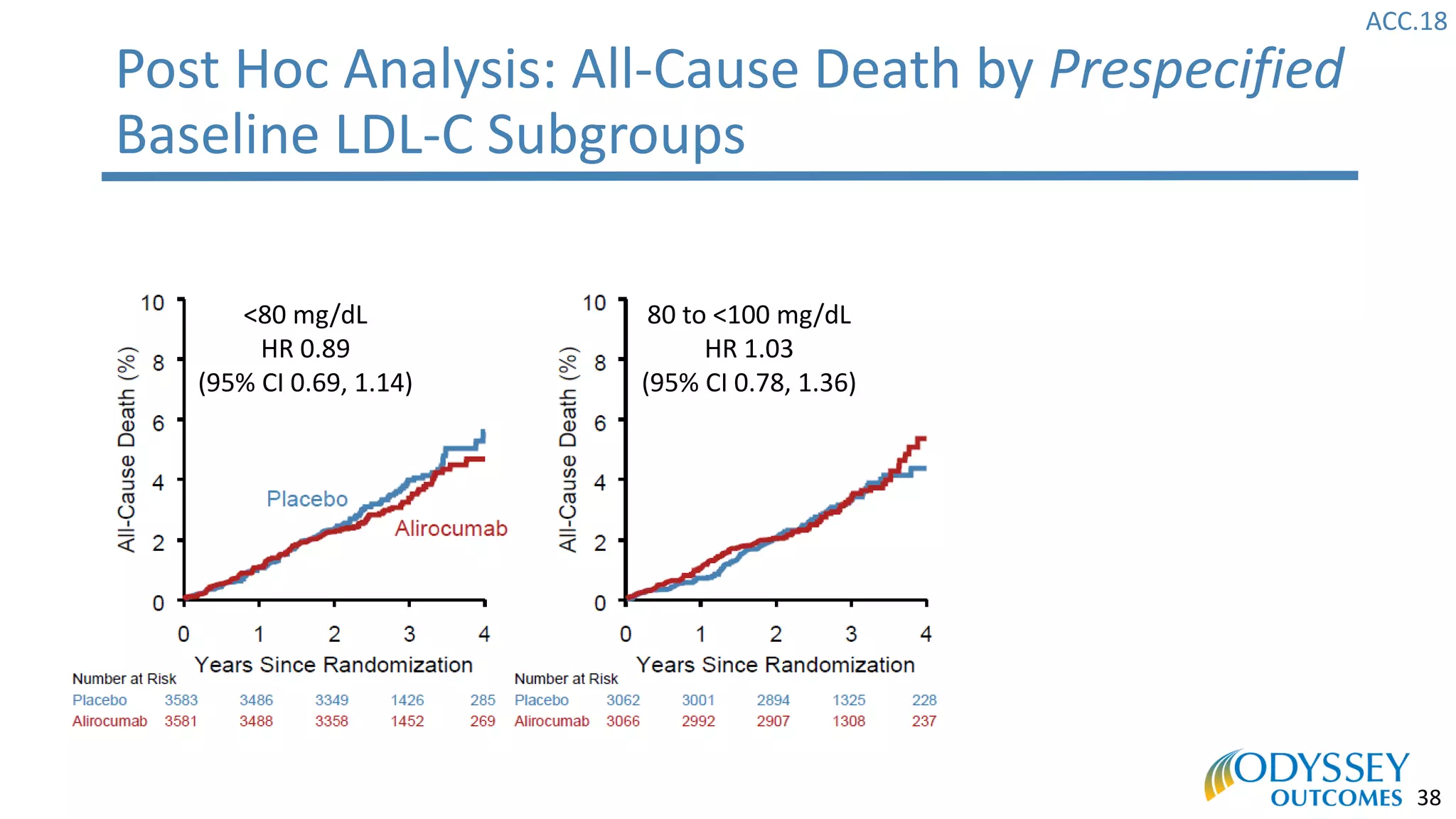
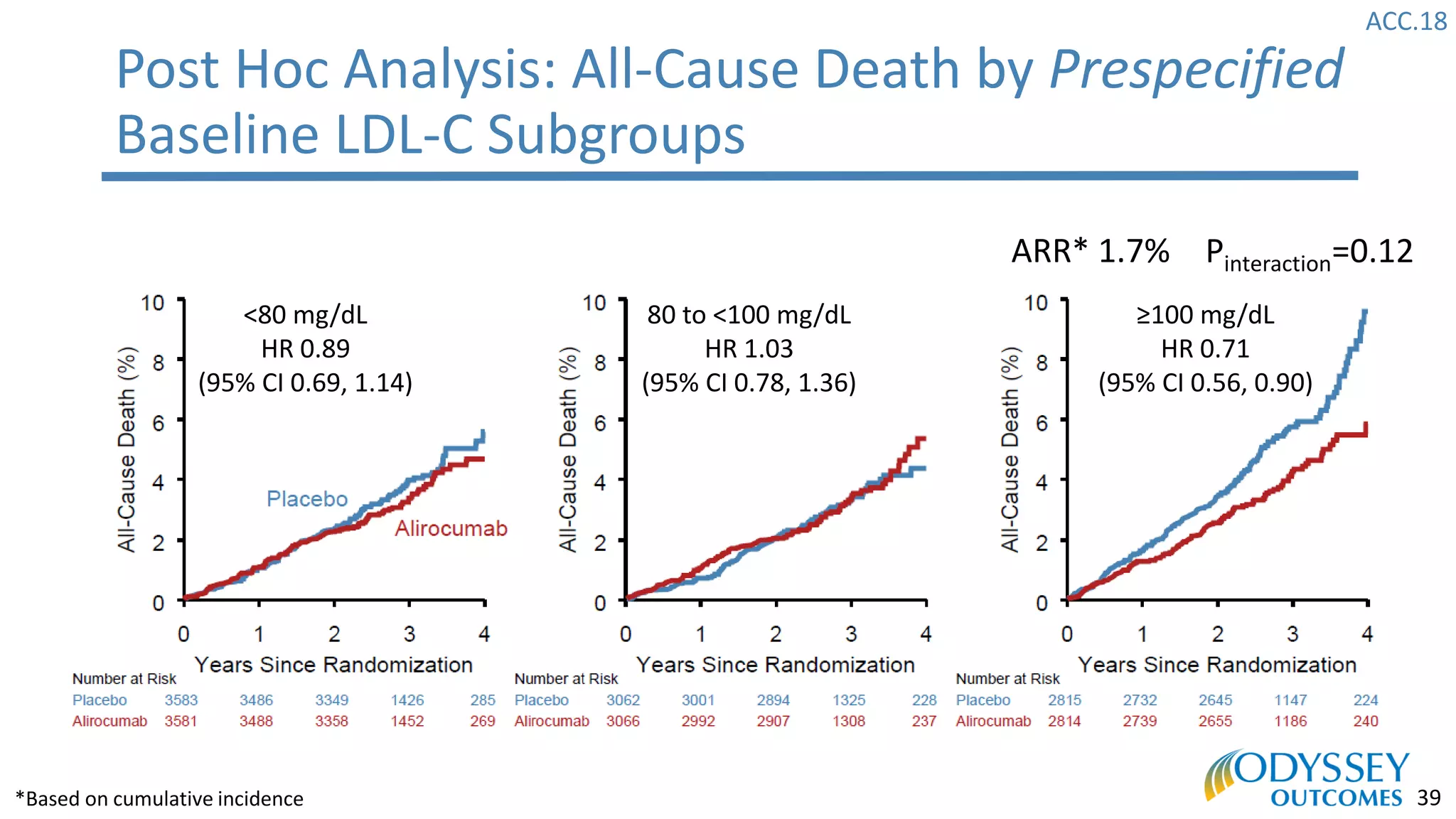
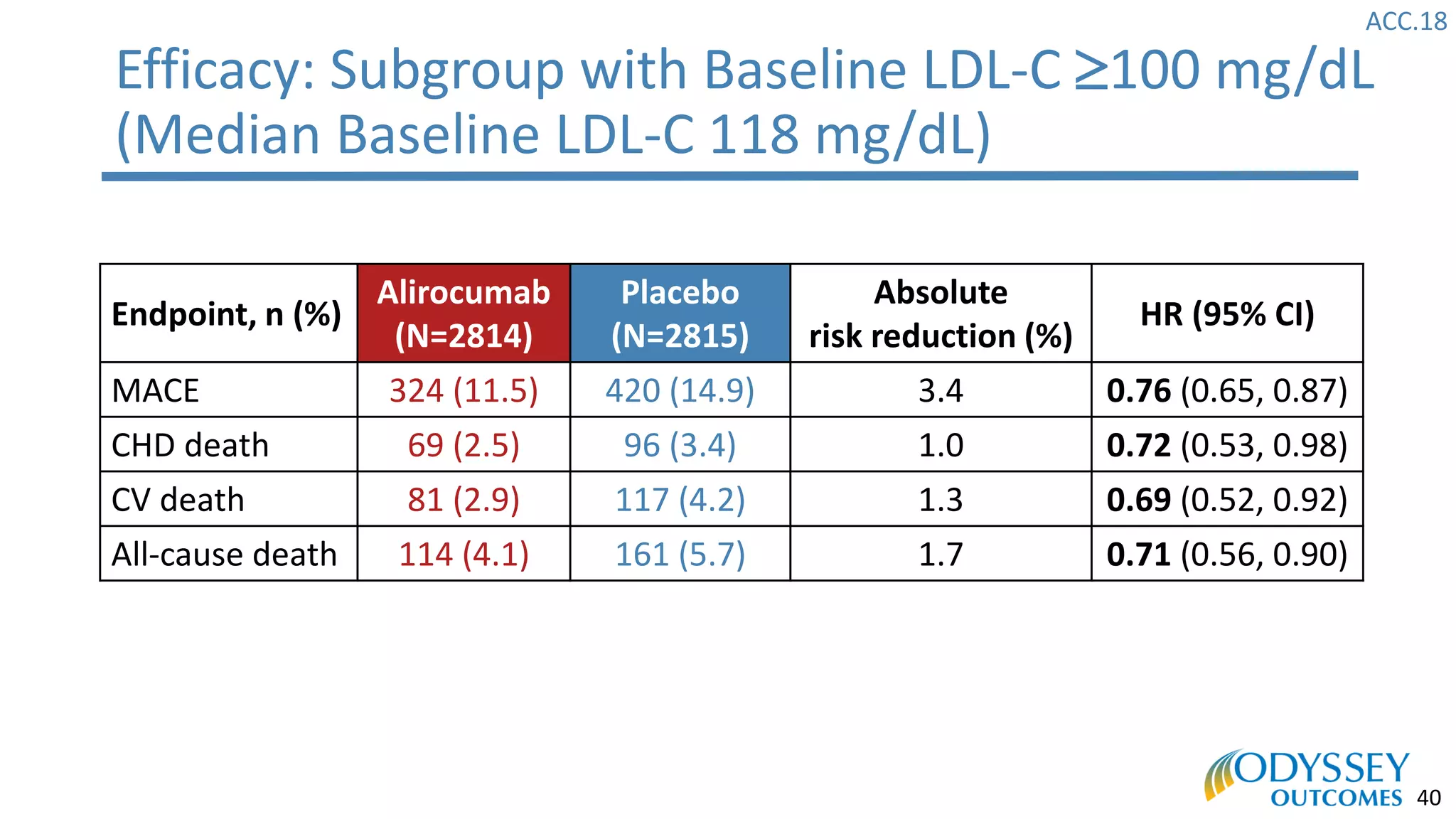
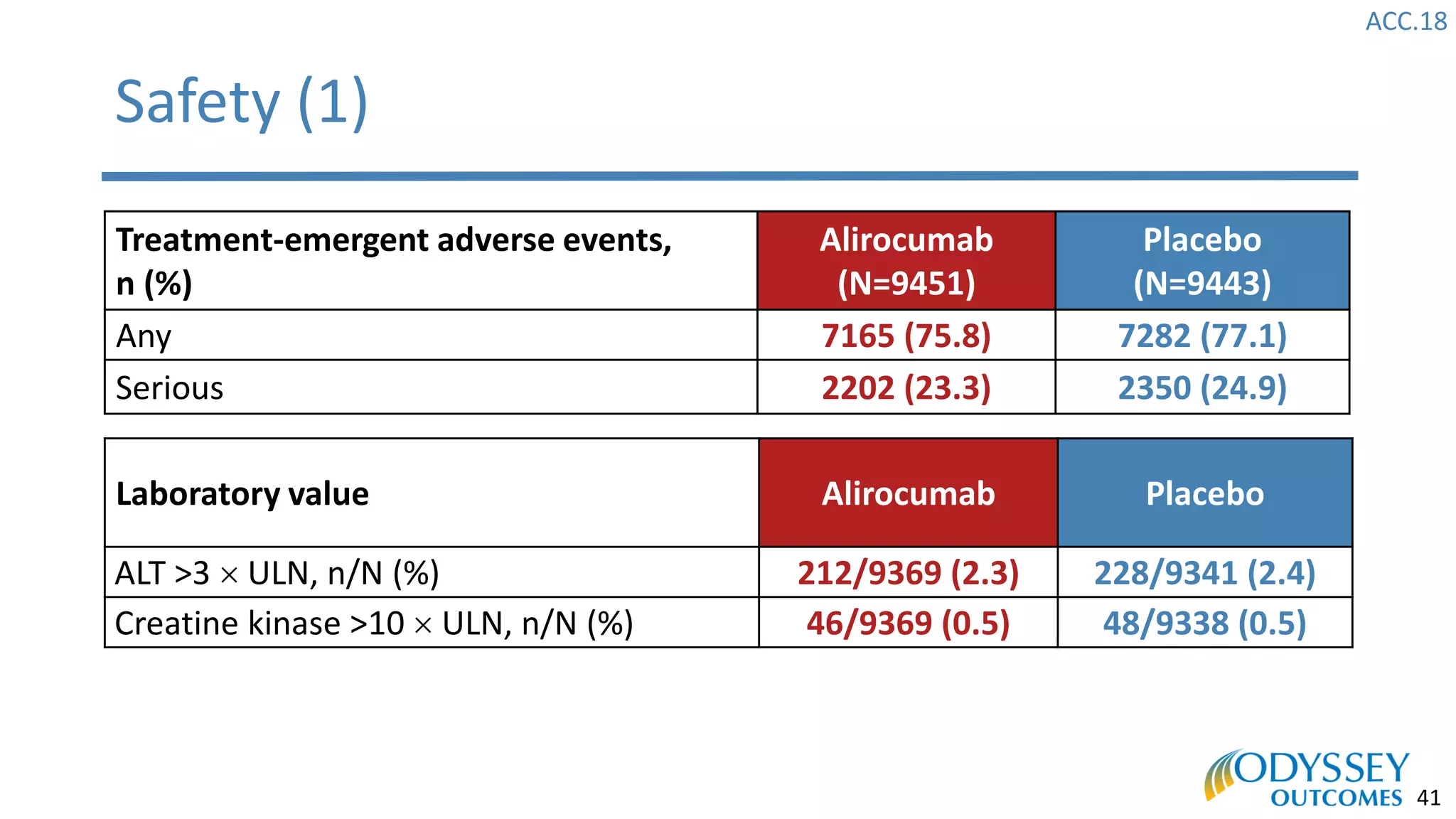
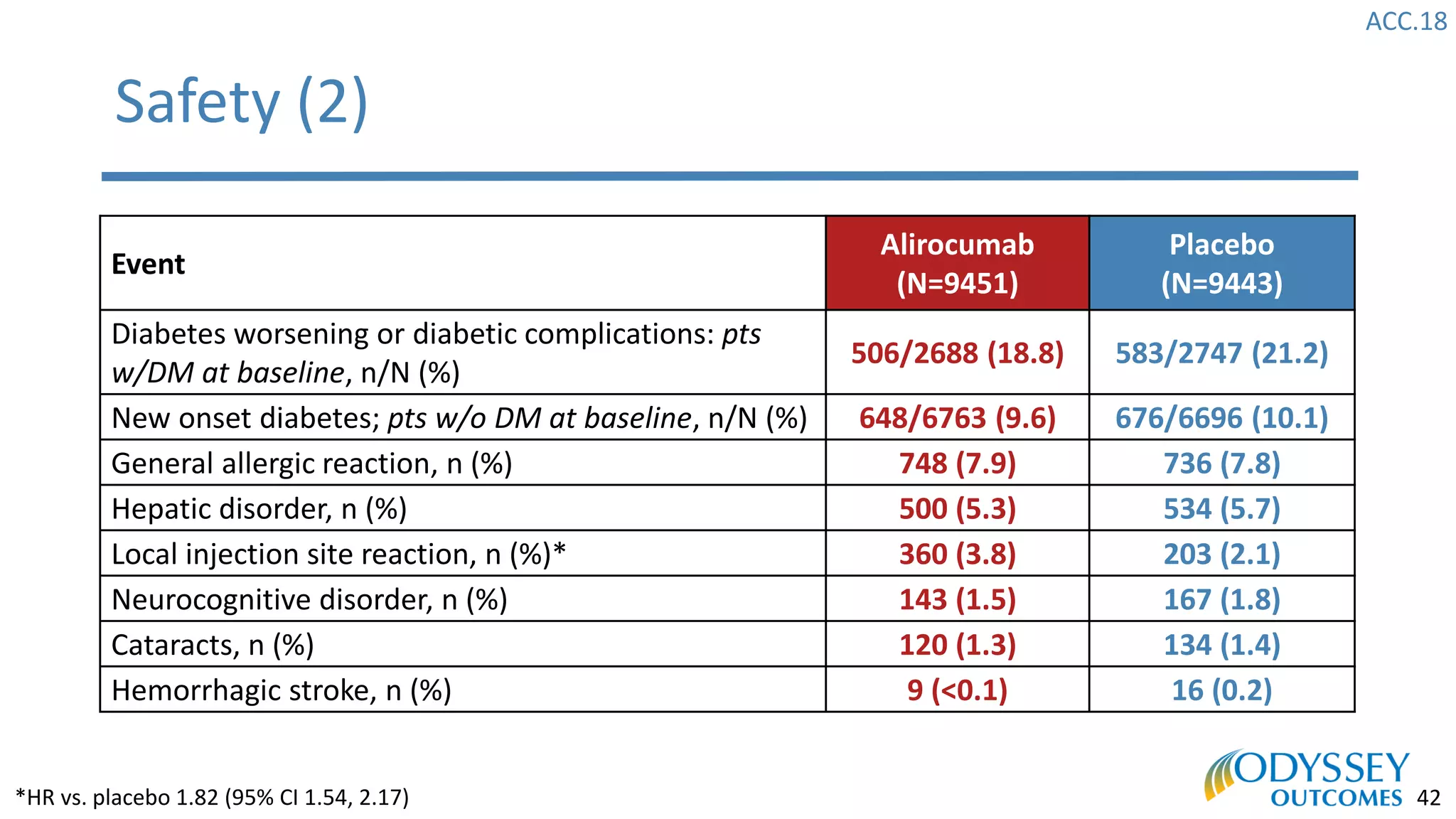
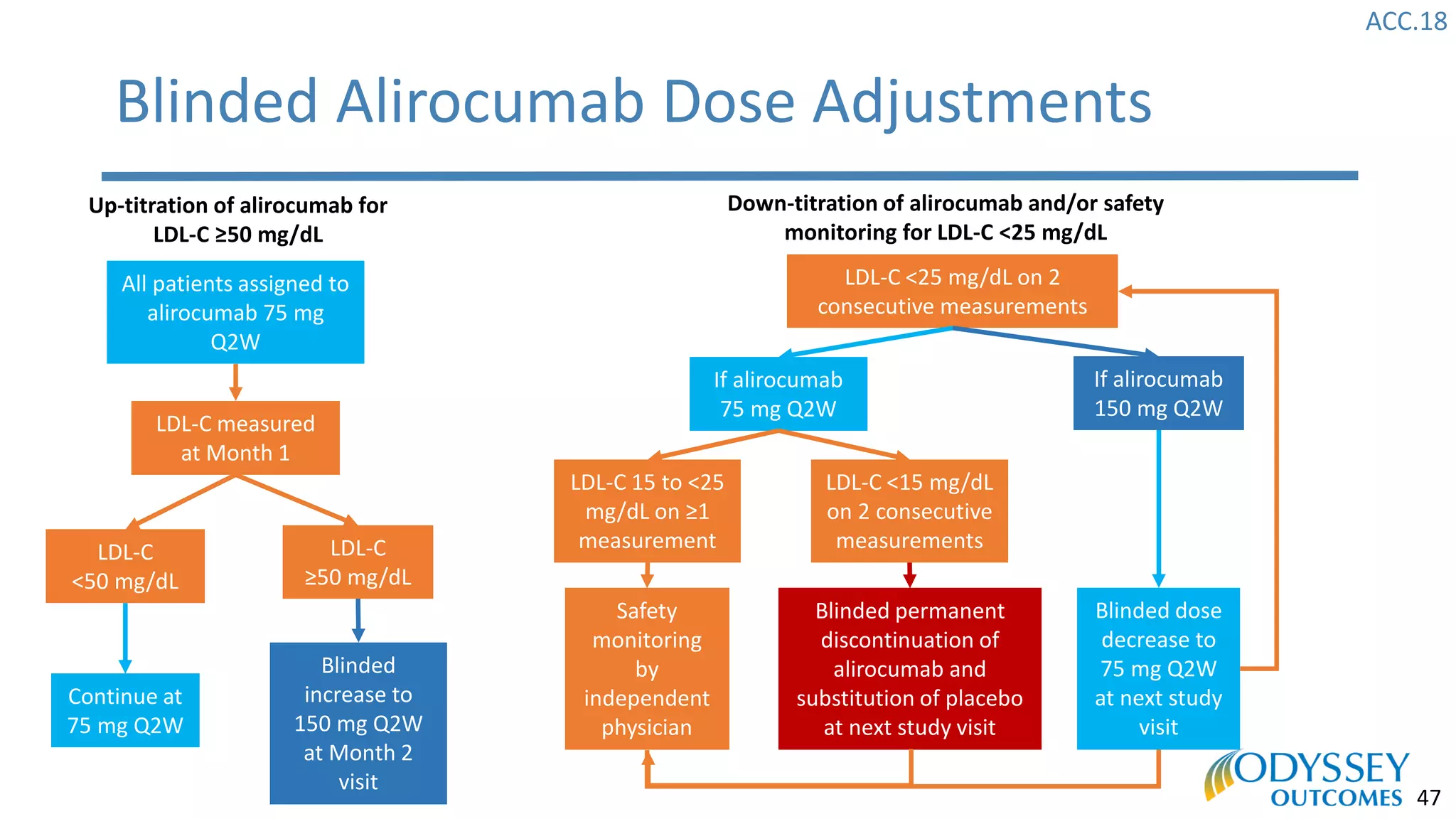
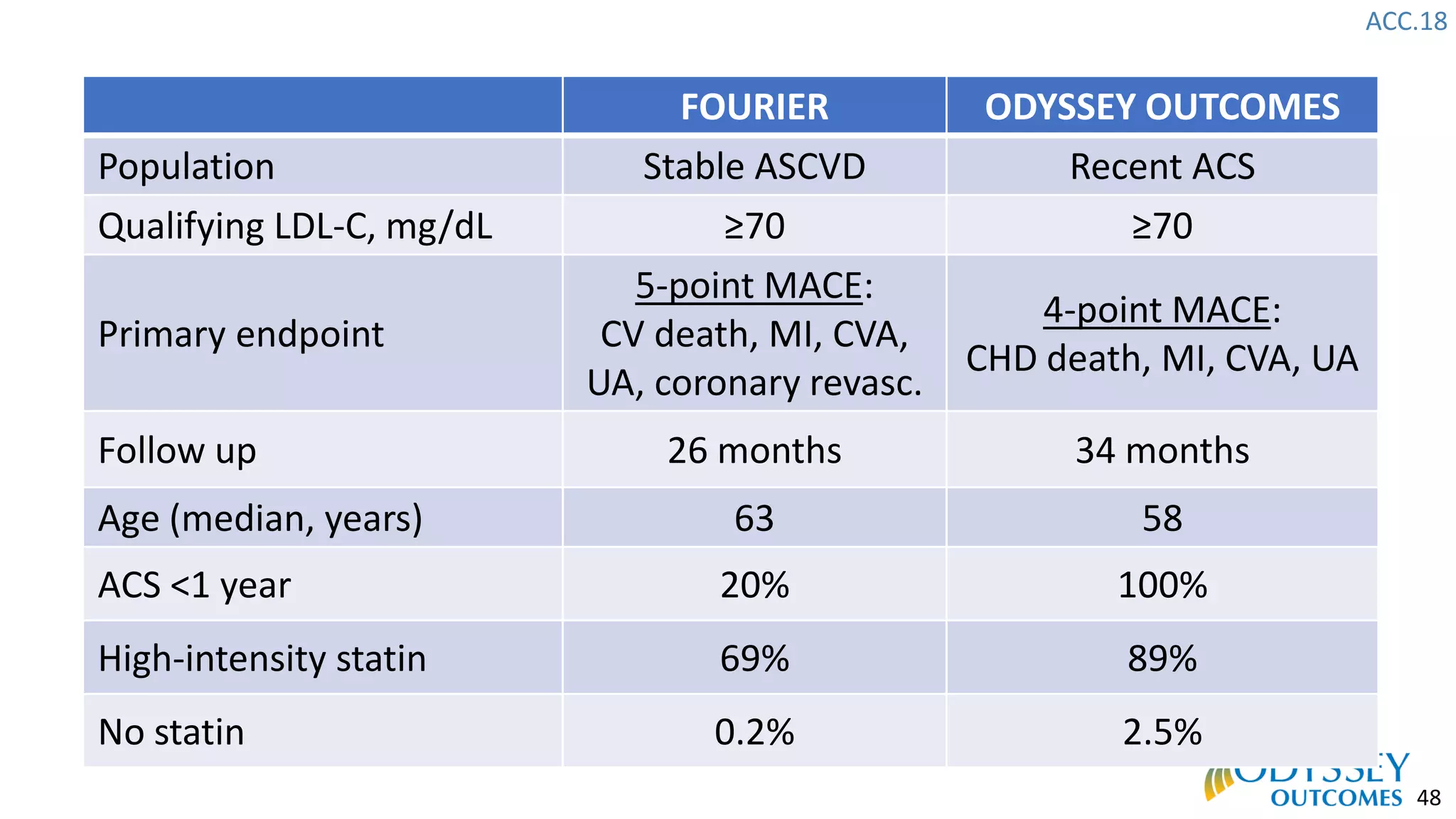
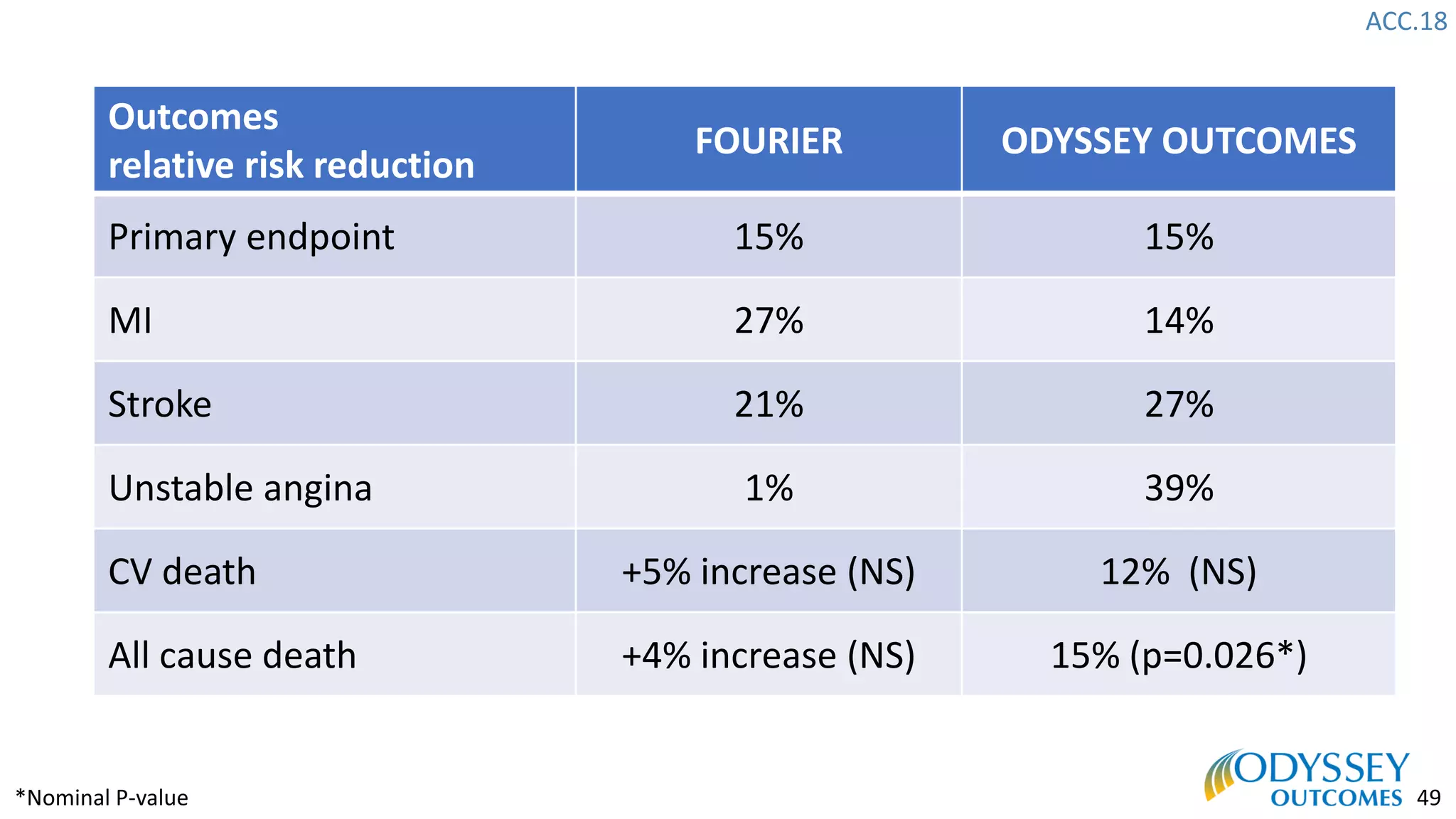
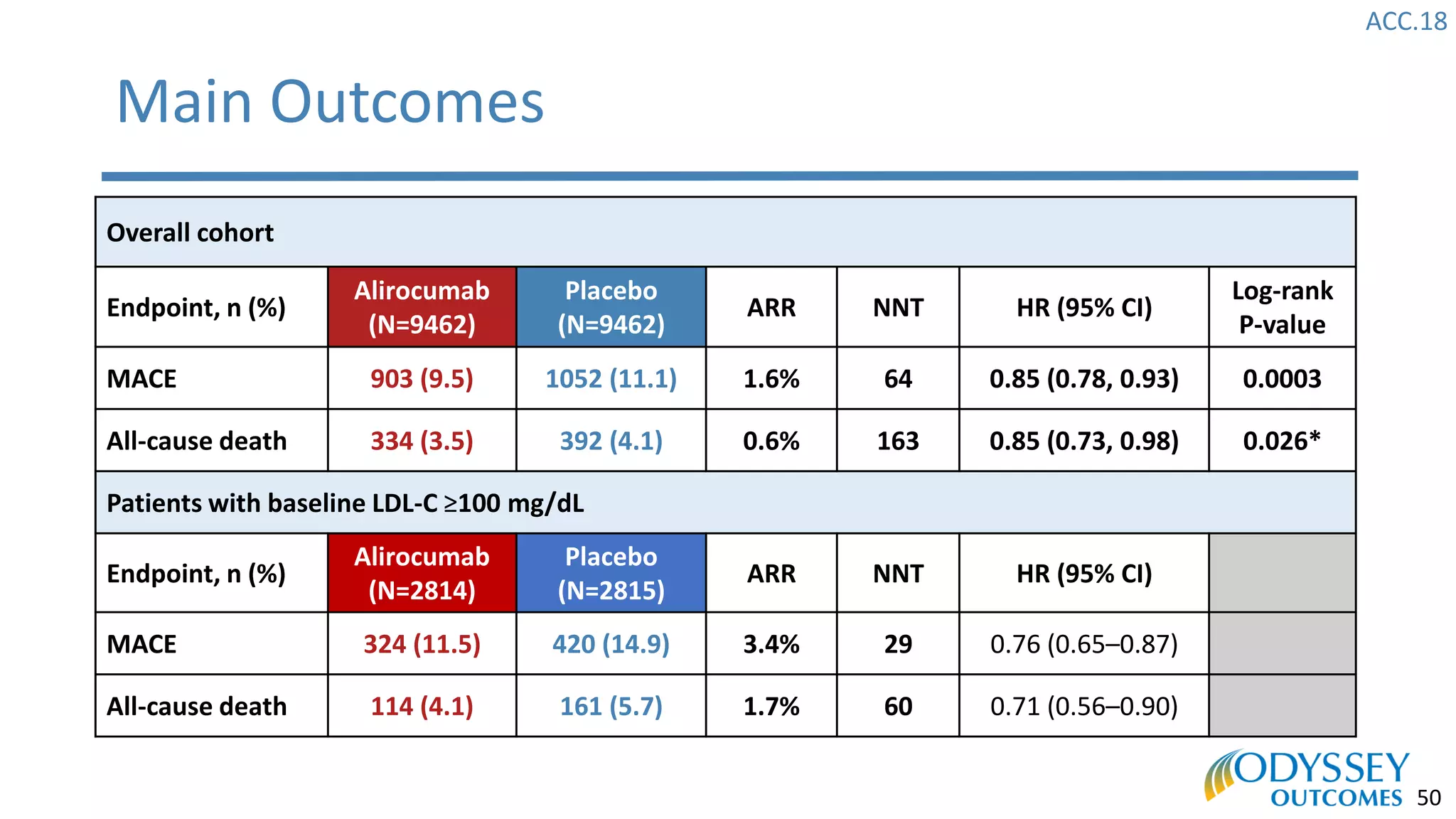
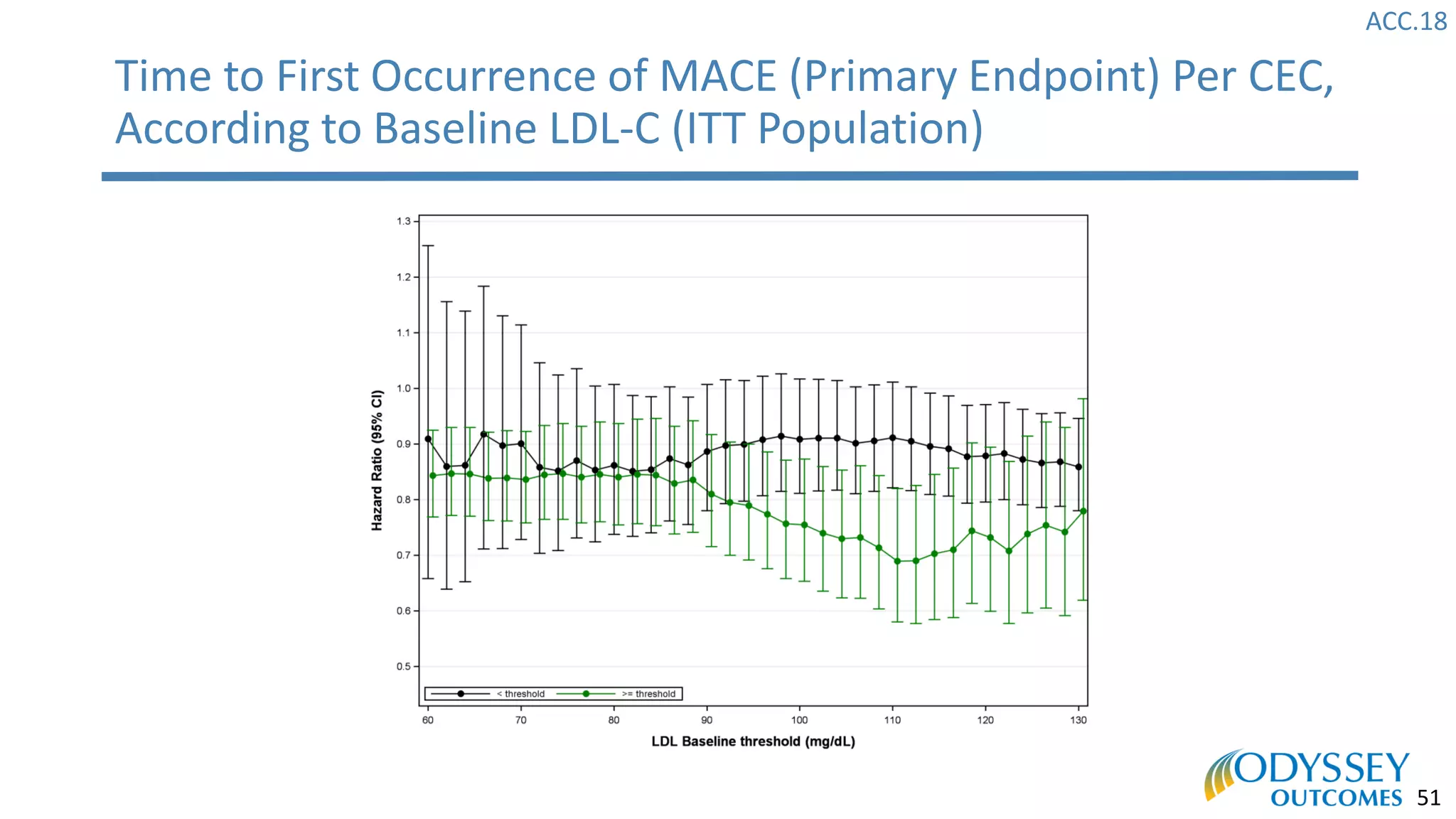
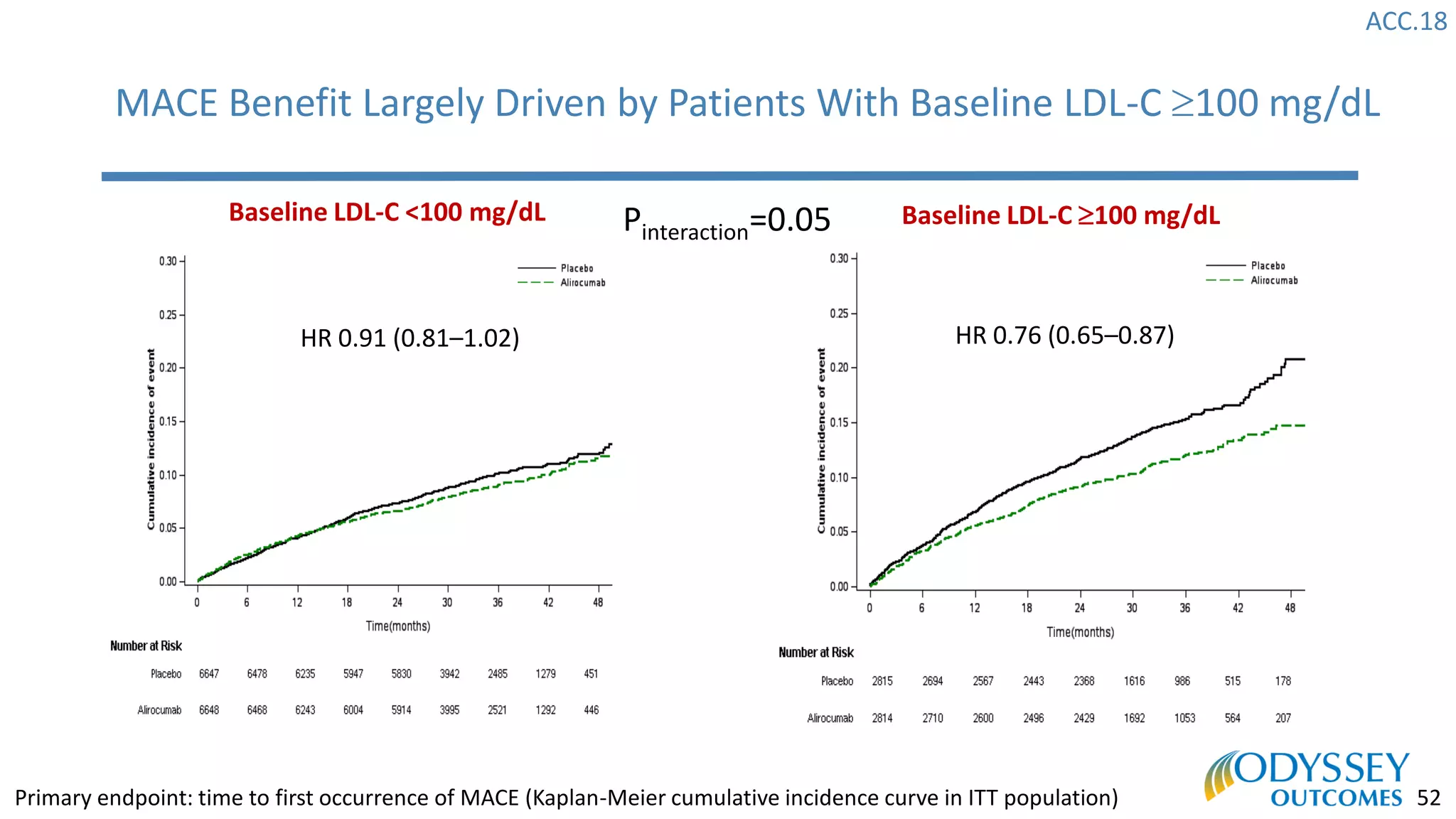
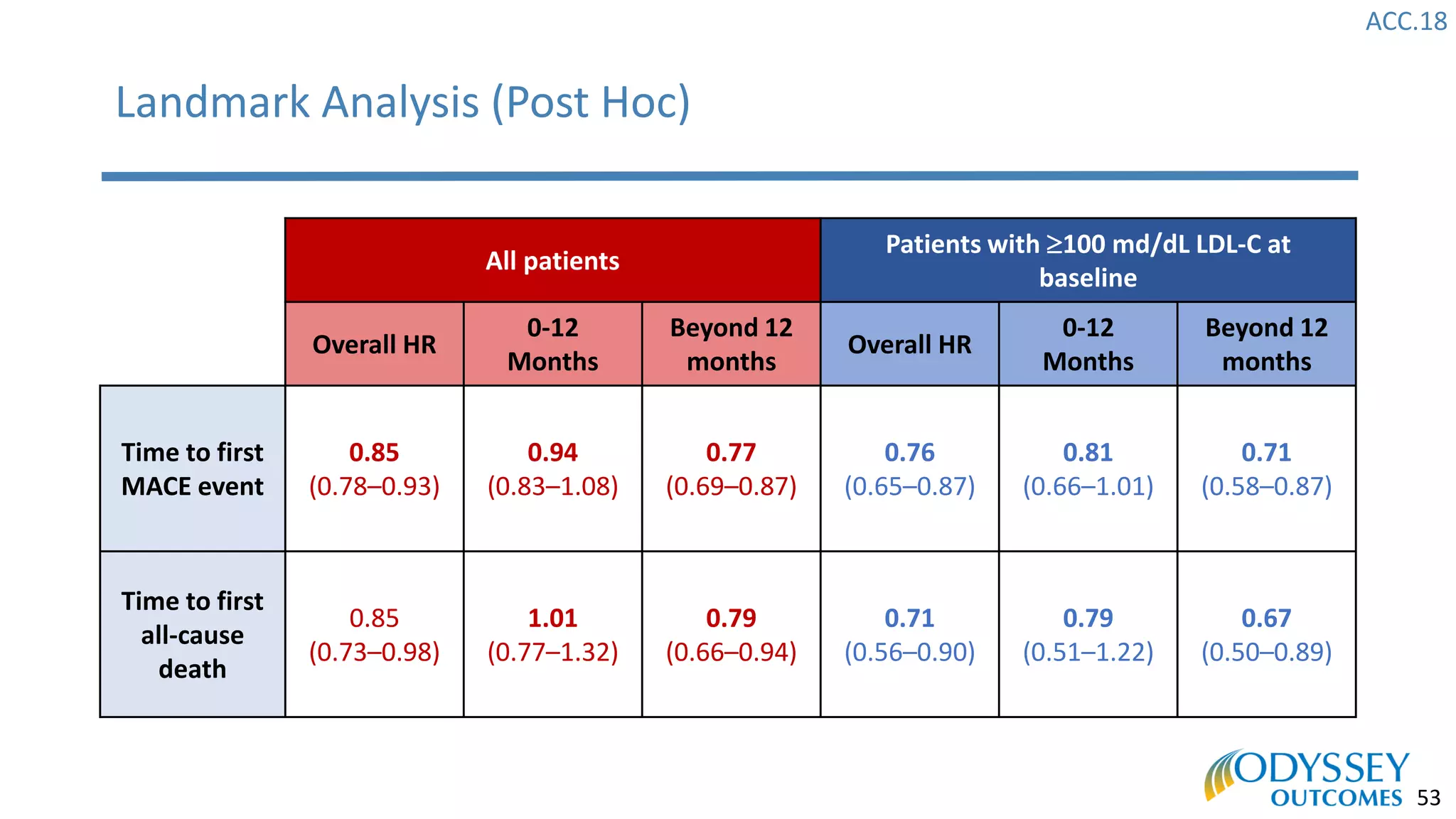
The Odyssey Outcomes trial investigated the efficacy of alirocumab, a monoclonal antibody targeting PCSK9, in reducing cardiovascular morbidity and mortality among patients post-acute coronary syndrome with elevated atherogenic lipoprotein levels despite maximum tolerated statin therapy. Conducted from 2012 to 2017 with nearly 19,000 participants across 57 countries, the trial aimed to determine if alirocumab could provide a significant benefit over placebo in this high-risk group. The study outlined stringent inclusion and exclusion criteria and emphasized its methodological rigor including independent statistical analyses and blinded treatment assignments.