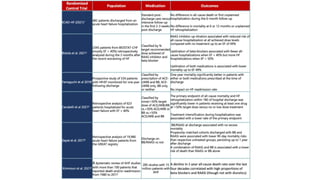
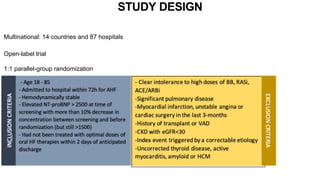
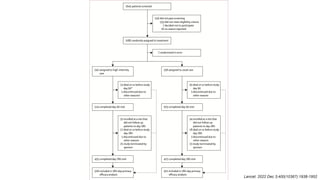
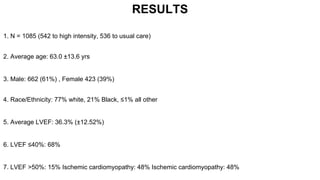
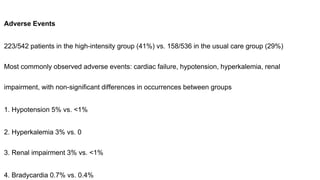
1) The STRONG-HF trial compared a high-intensity follow-up approach focused on rapid up-titration of guideline-directed medical therapies (GDMT) for heart failure to target doses within 90 days of hospital discharge to standard post-discharge care.
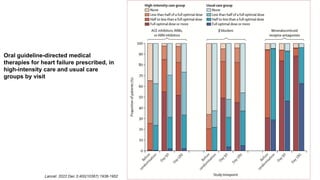
2) The high-intensity approach resulted in significantly higher rates of patients reaching target doses of renin-angiotensin-aldosterone inhibitors, beta-blockers, and mineralocorticoid receptor antagonists by 90 days compared to standard care.
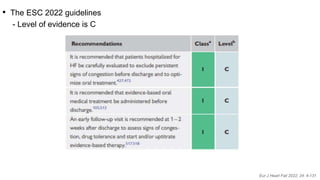
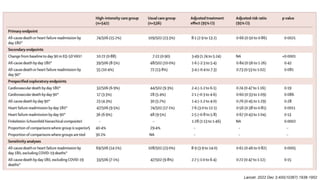
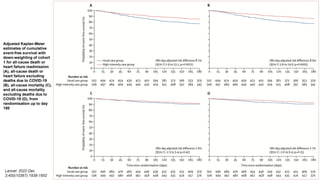
3) At 180 days, the high-intensity approach reduced the combined outcome of all-cause death or heart failure readmission compared to standard care and improved quality of life, with no