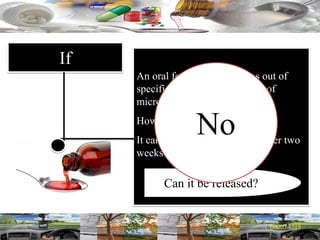
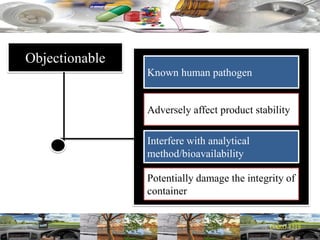
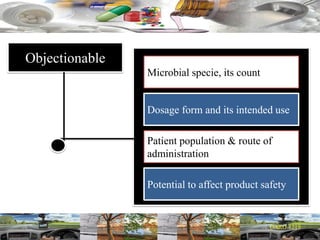
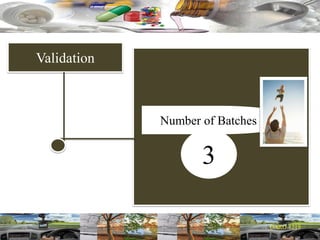
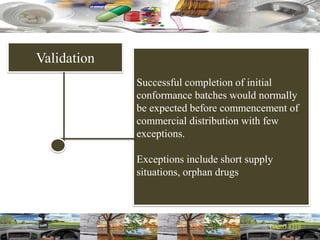
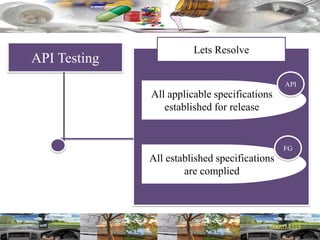
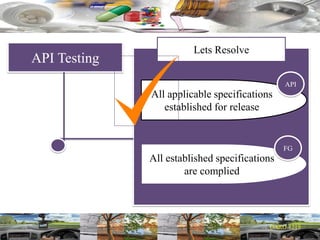
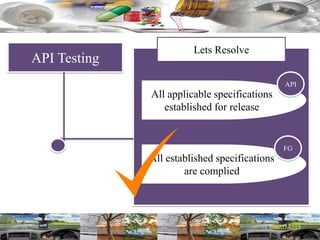
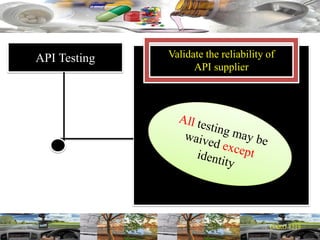
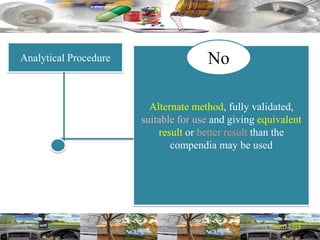
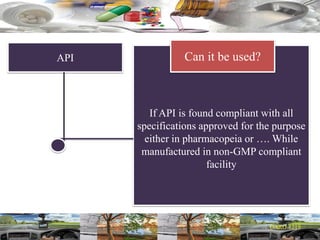
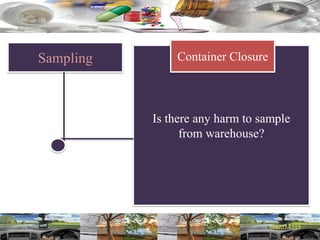
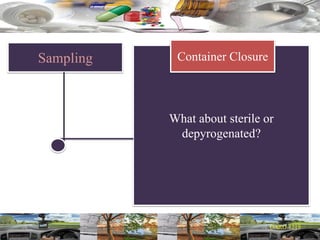
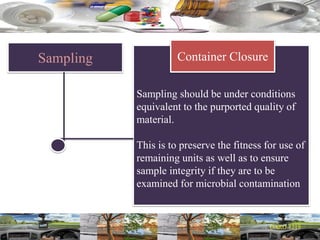
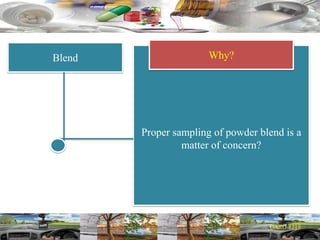
This document provides a summary of a dashboard and reflective review presentation from 2019. It discusses various quality topics related to pharmaceutical manufacturing including assay testing of APIs, use of preservatives, microbial limits, process validation, cleaning validation, API testing responsibilities, and more. Key points emphasized include using a risk-based approach to assessing changes and product holds, ensuring sampling methods are scientifically sound, and the importance of evaluating cleaning procedures to maintain ability to clean. Recent regulatory guidance from EMA regarding evaluation of nitrosamines is also summarized.