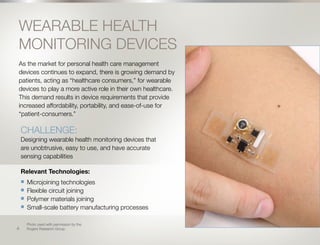
The global medical device industry, valued at $350 billion, is projected to grow at a 4.9% CAGR from 2015-2020, driven by trends such as an aging population, personalized treatment, and wearable health devices. Key challenges include adapting to rapid technological advancements, meeting regulatory demands, and developing cost-effective solutions for emerging markets. Manufacturers must innovate in areas such as miniaturization, quality inspection, and technology fusion to capitalize on these trends and maintain competitive advantages.