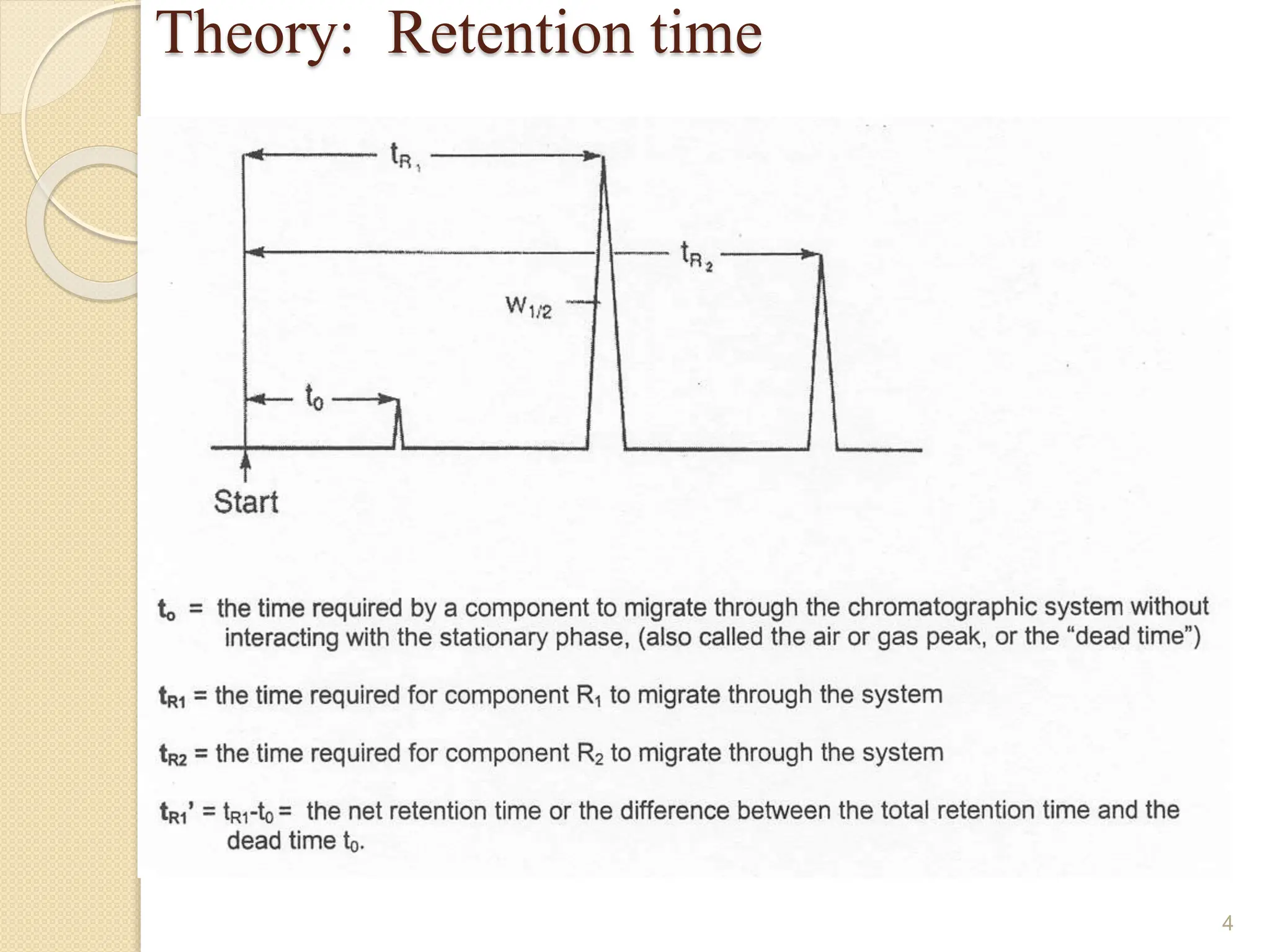
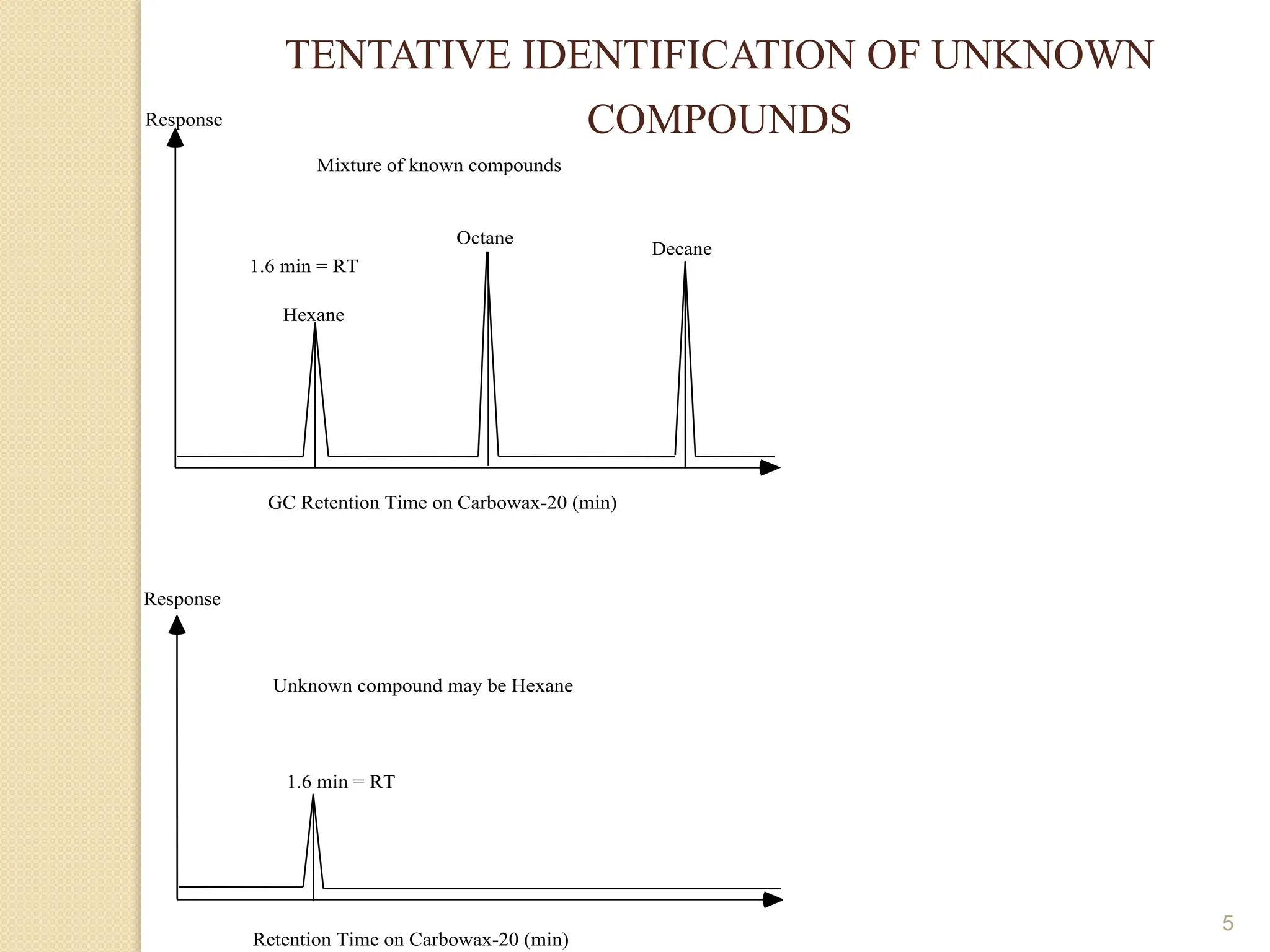
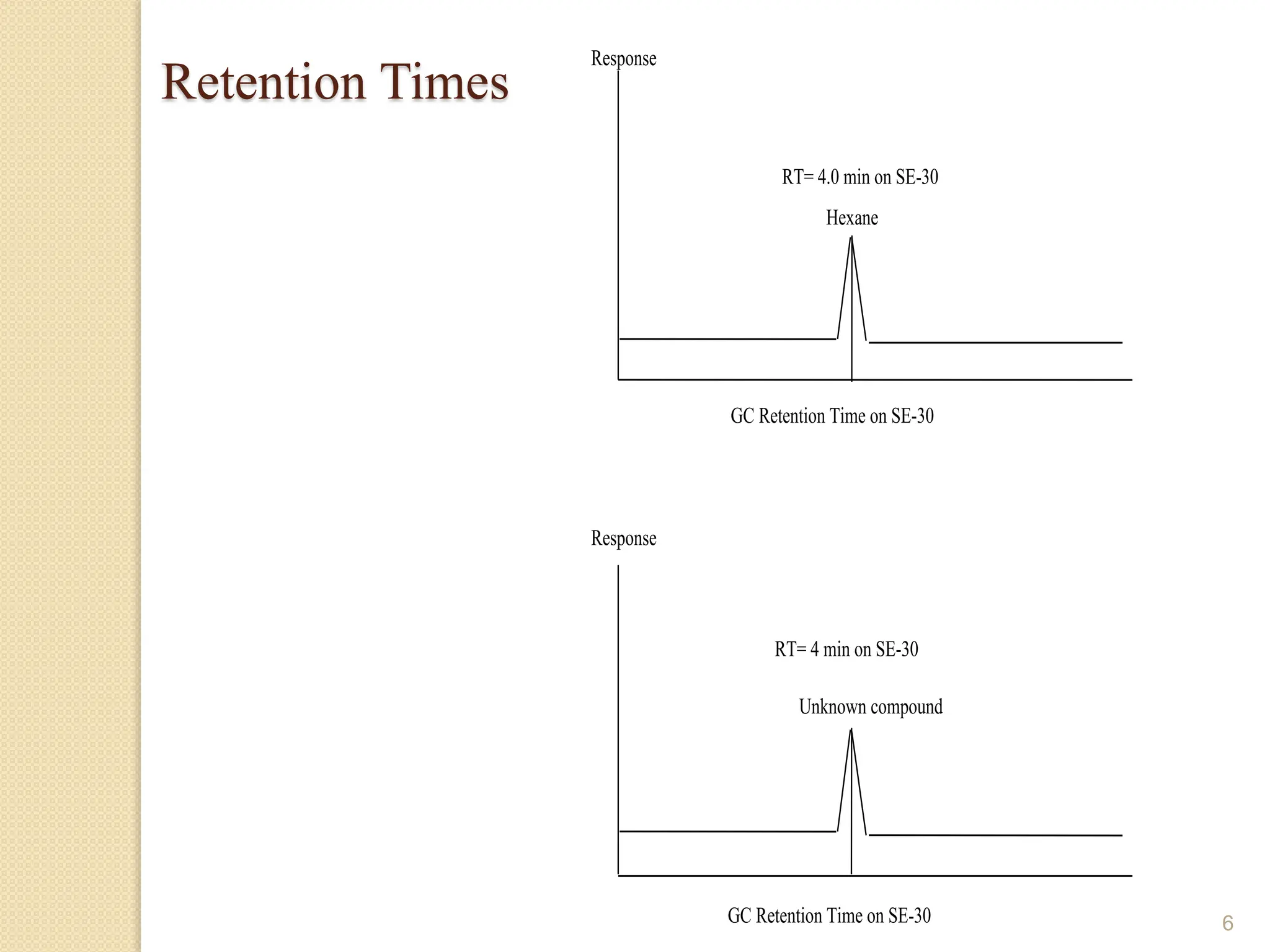
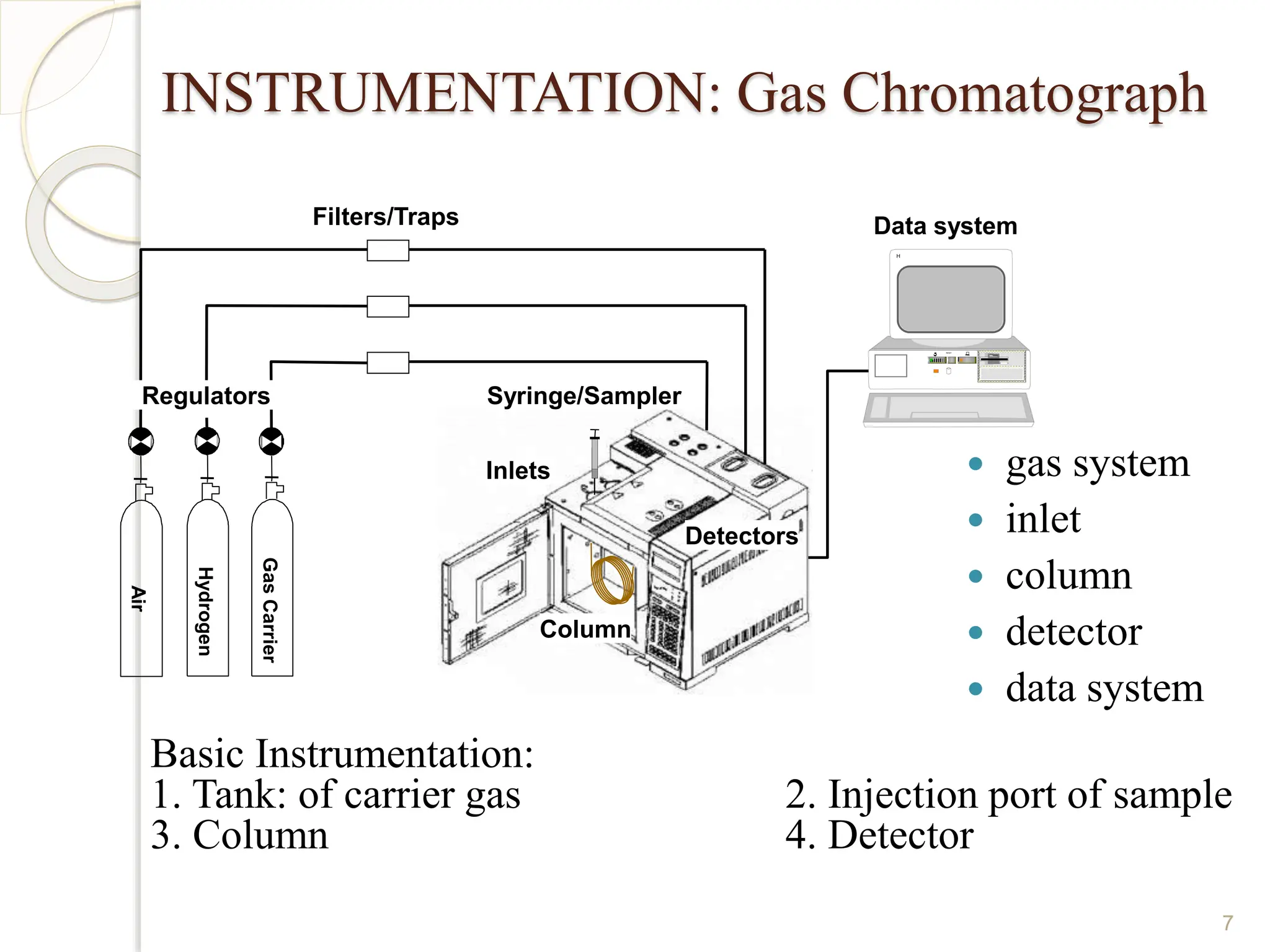
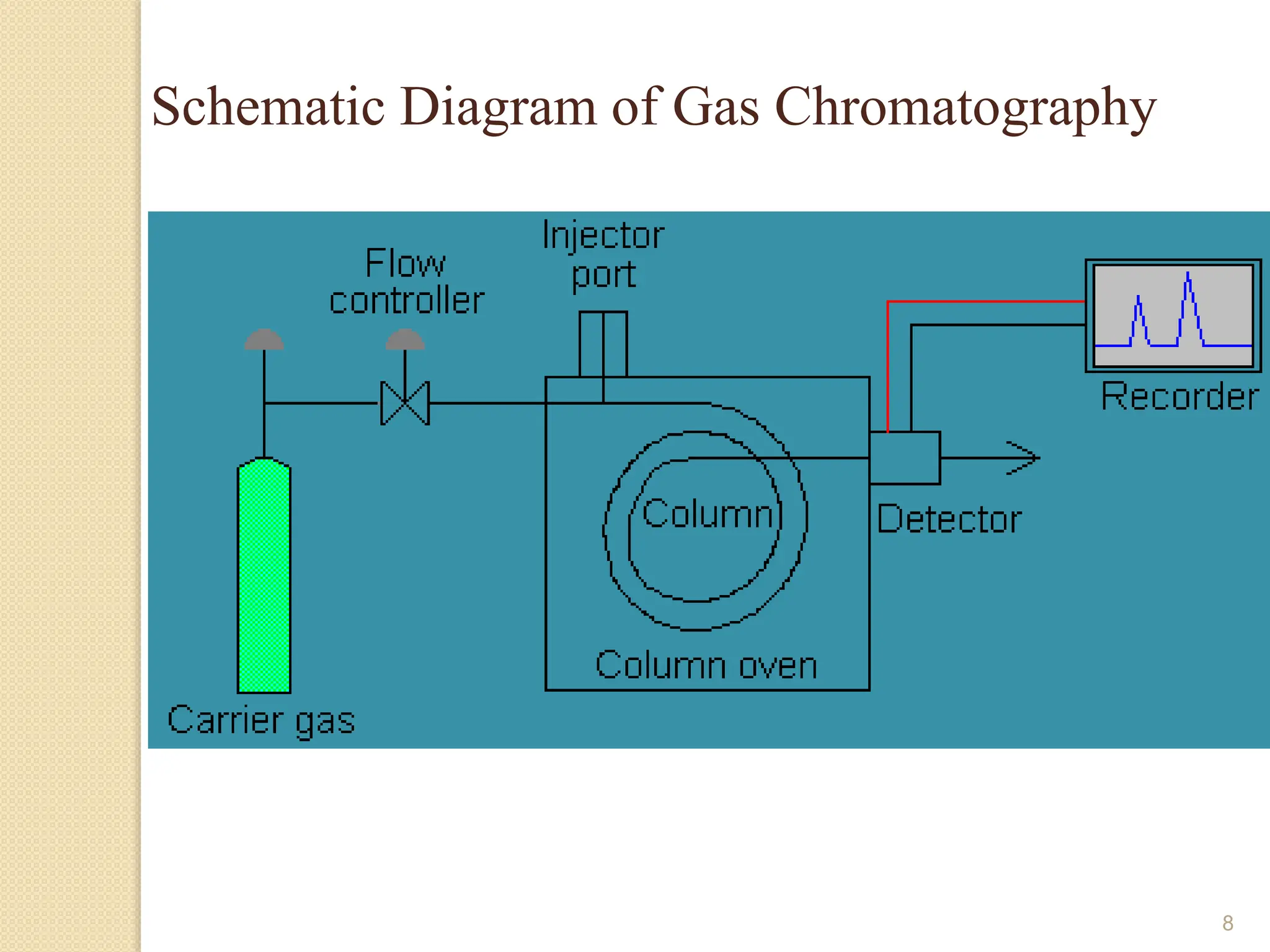
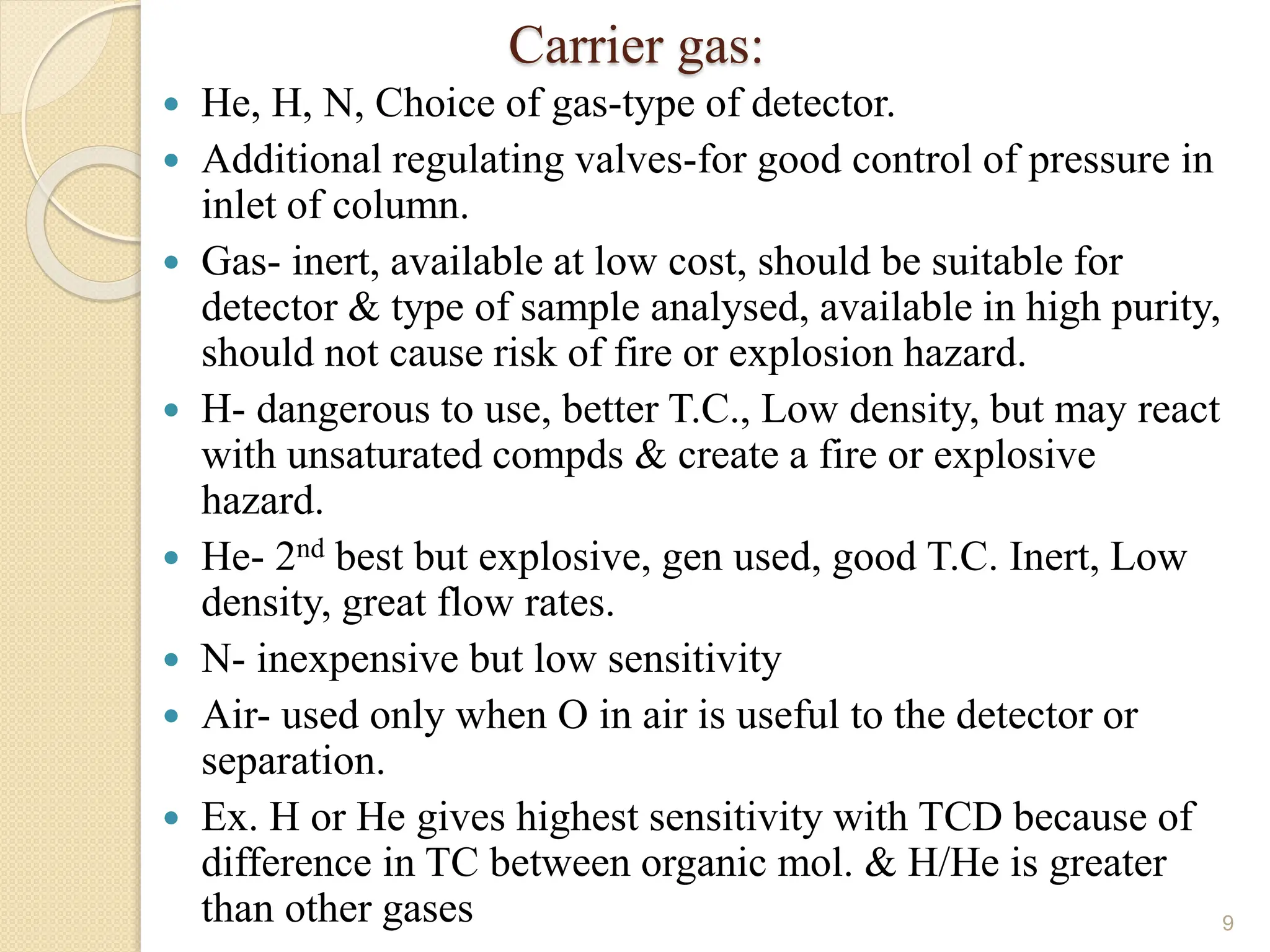
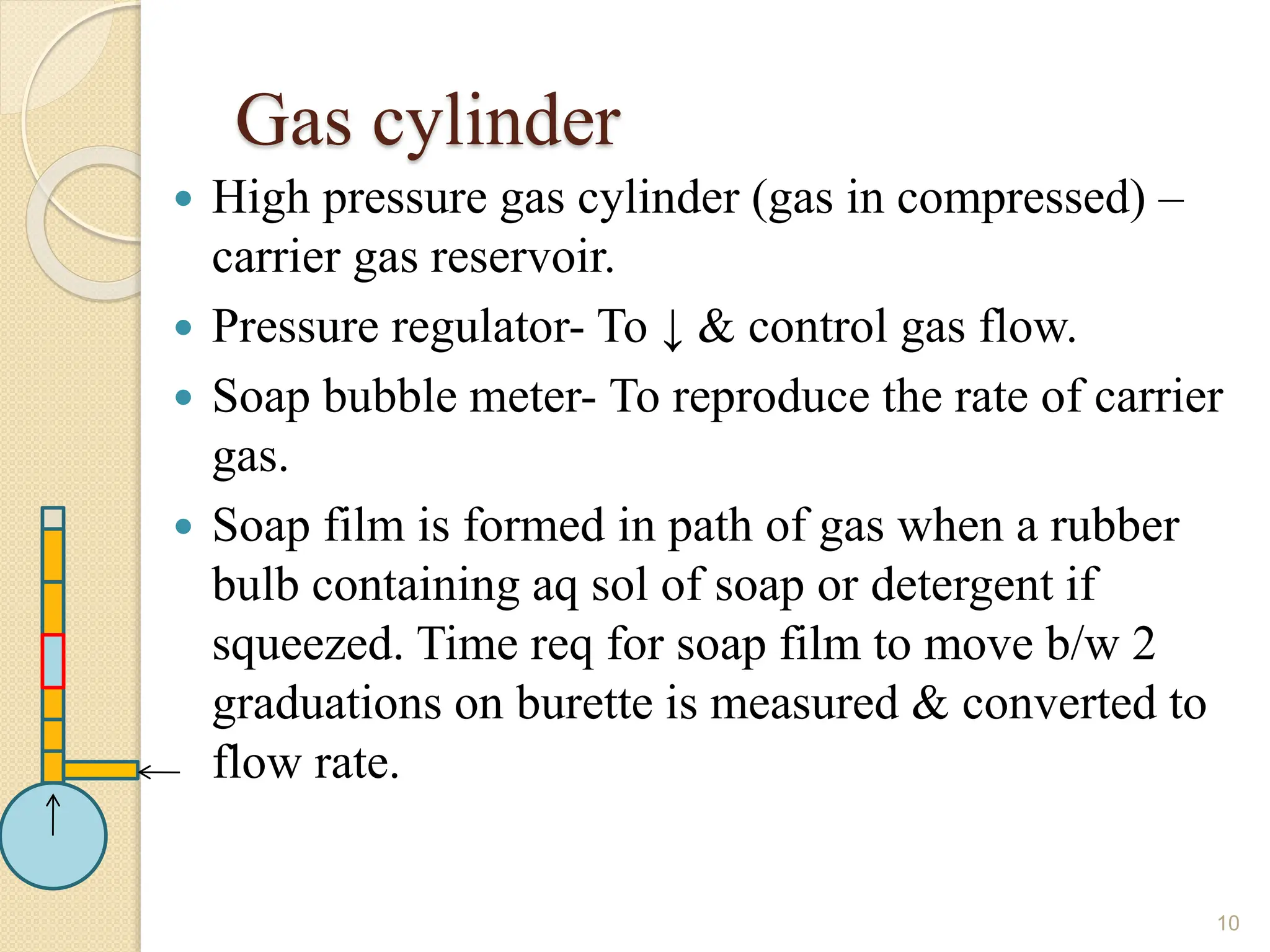
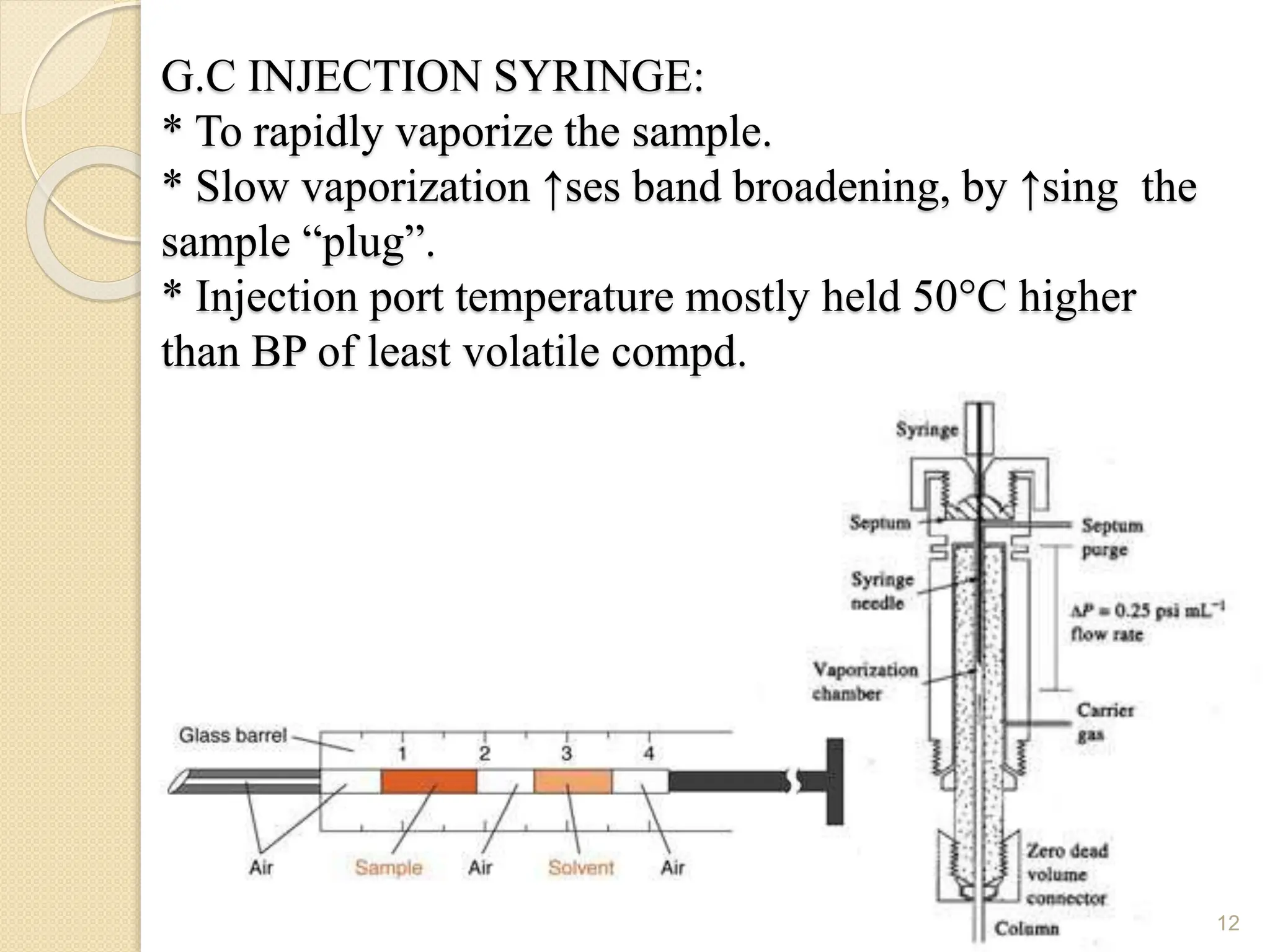
This document provides an overview of gas chromatography, detailing its history, theory, instrumentation, and applications. It discusses both gas-solid chromatography (GSC) and gas-liquid chromatography (GLC), highlighting GLC as the more widely used method for analyzing volatile substances. Additionally, it outlines the role of carrier gases, sample introduction techniques, and the importance of maintaining proper temperature during the analysis.