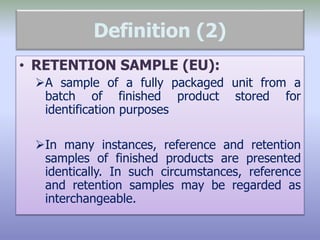
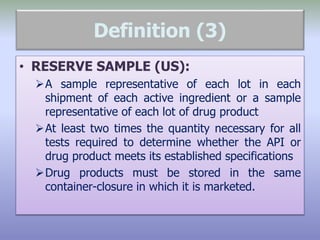
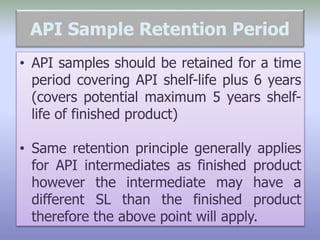
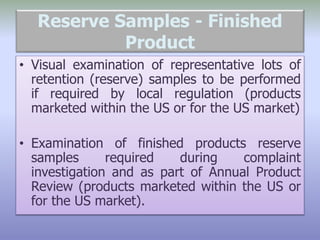
This document defines reference, retention, and reserve samples and provides requirements for storing and retaining these samples. A reference sample is stored for analyzing if needed during a batch's shelf life. A retention sample is a fully packaged finished product unit stored for identification. A reserve sample is representative of each lot shipped and is twice the quantity needed for testing. Samples must be retained for various time periods depending on the material and local regulations. Storage conditions must be consistent with product specifications and limited to authorized personnel.