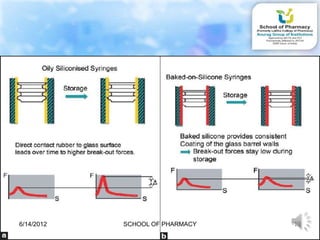
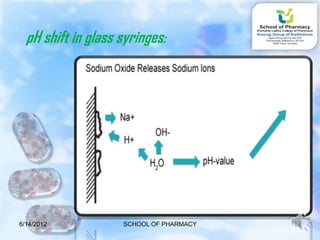
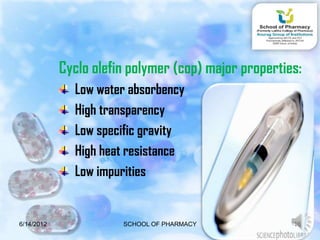
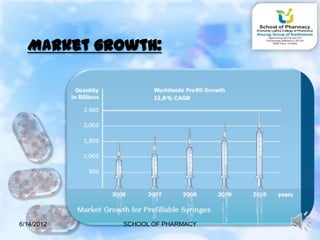
The document discusses prefilled syringes. It describes prefilled syringes as single-dose packets of injectable drugs that have a pre-attached needle. It discusses the purpose of prefills for primary packaging and drug delivery. It also outlines the types of prefill systems using glass or plastic, describes common materials used, and the filling and sterilization processes. The document provides advantages like convenience and accuracy as well as some disadvantages. It also discusses factors driving the growth of prefilled syringes.