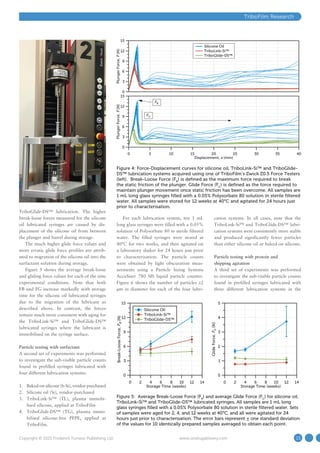
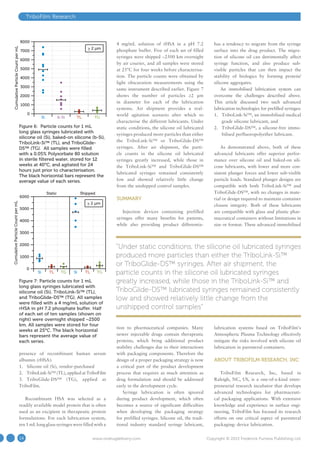
This document discusses lubrication challenges with prefilled syringes and introduces TriboFilm Research's solutions. Silicone oil is commonly used to lubricate prefilled syringes but can migrate, causing inconsistent plunger forces and introducing particles into drugs. TriboFilm has developed an atmospheric plasma technology that can immobilize lubricants like silicone oil or its PFPE lubricant onto syringe surfaces, reducing lubricant migration issues. This plasma process crosslinks the lubricant during syringe manufacturing without contaminants.