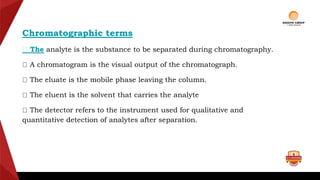
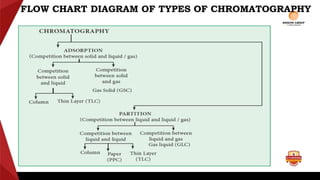
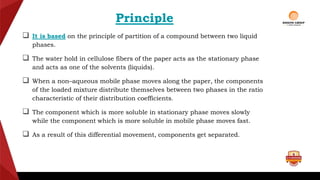
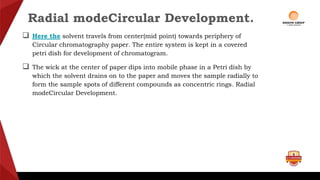
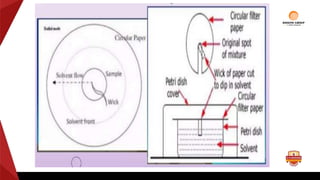
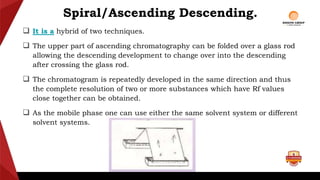
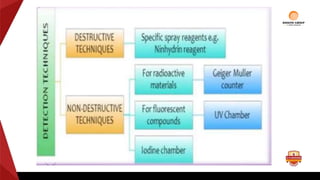
Paper chromatography is a type of planar chromatography that uses paper as the stationary phase. It involves separating mixtures based on how compounds partition between the stationary phase (cellulose fibers in the paper) and a mobile liquid phase. When a sample mixture is applied to the paper and an appropriate solvent is run up the paper, the different compounds will travel different distances depending on how they partition between the paper and solvent. Their positions can then be detected visually or through chemical detection methods to separate and analyze the compounds in the original mixture. Paper chromatography is useful for separating and analyzing colored pigments, performing qualitative analyses, and examining complex mixtures in fields like pathology and forensics.