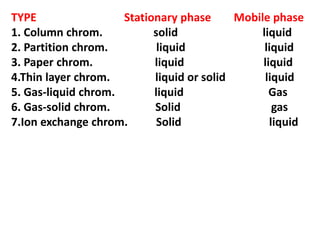
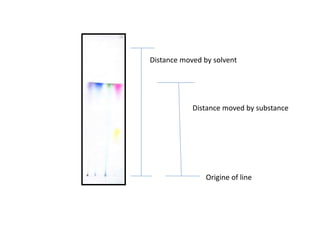
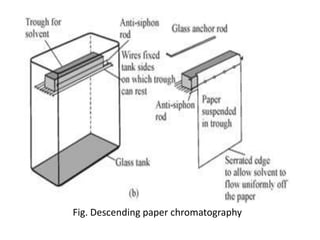
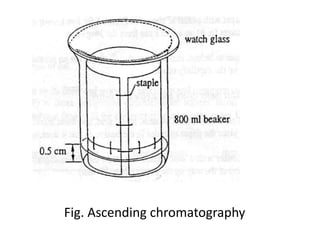
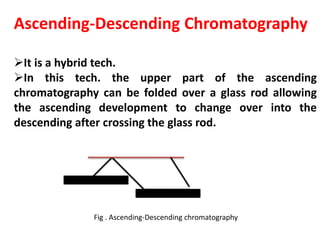
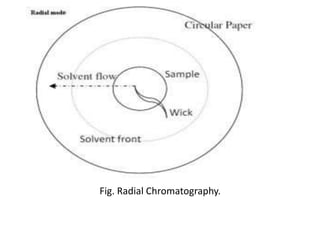
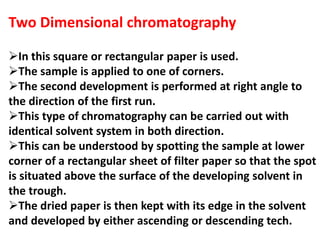
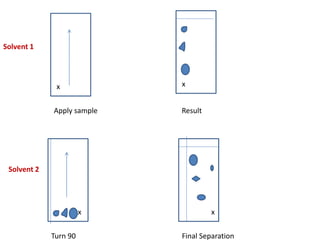
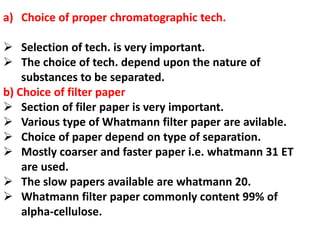
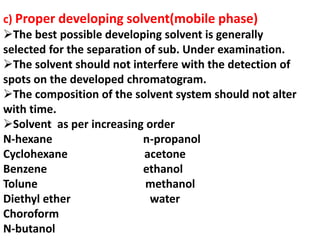
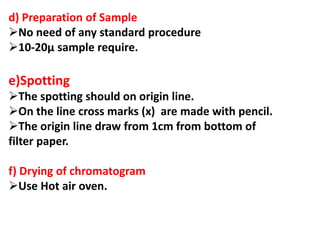
Chromatography is a technique used to separate components of a mixture. It works by distributing the components between two phases - a stationary phase and a mobile phase. Paper chromatography is a type of chromatography where the stationary phase is filter paper and the mobile phase is a liquid solvent. Key steps in paper chromatography include choosing a suitable filter paper and solvent, preparing and spotting the sample, developing the chromatogram by allowing the solvent to travel up or down the paper, visualizing the separated components, and calculating Rf values to identify substances.