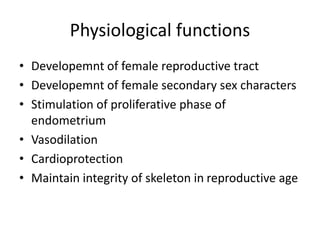
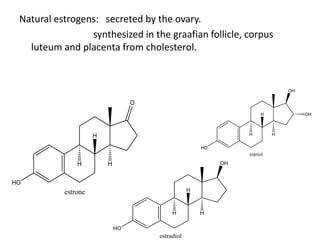
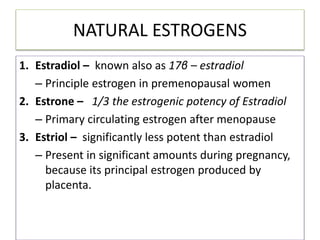
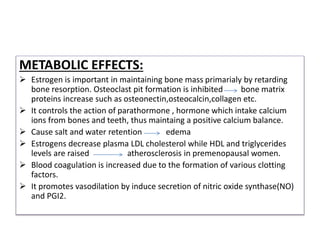
Estrogen has several physiological functions including the development of female reproductive tract and secondary sex characteristics. There are natural estrogens like estradiol, estriol, and estrone that are secreted by the ovaries, and synthetic estrogens like ethinylestradiol and diethylstilbestrol that have longer durations of action. Estrogen receptors mediate their effects, and their actions include effects on sex organs like stimulating the uterus and vagina, metabolic effects like maintaining bone mass and calcium balance, and therapeutic uses like hormone replacement therapy and osteoporosis treatment. Common adverse effects of estrogen therapy include nausea, breast tenderness, and increased risks of certain cancers.