Report
Share
More Related Content
What's hot
What's hot (20)
Types of reaction by Muhammad Fahad Ansari 12IEEM14

Types of reaction by Muhammad Fahad Ansari 12IEEM14
4th Lecture on Elements of groups 16, 17 & 18 | Chemistry Part I | 12th Std

4th Lecture on Elements of groups 16, 17 & 18 | Chemistry Part I | 12th Std
Viewers also liked
Viewers also liked (6)
Similar to Redox o h
Similar to Redox o h (20)
Ncert class 10 - science - chapter 1 - chemical reactions and equations

Ncert class 10 - science - chapter 1 - chemical reactions and equations
CBSE CLASS 10 CHEMISTRY CHAPTER 1 CHEMICAL REACTIONS AND EQUATIONS PART 2 TYP...

CBSE CLASS 10 CHEMISTRY CHAPTER 1 CHEMICAL REACTIONS AND EQUATIONS PART 2 TYP...
Oxidation – reduction reactions by Muhammad Fahad Ansari 12IEEM14

Oxidation – reduction reactions by Muhammad Fahad Ansari 12IEEM14
Redox Reactions. Chemical Reactions occurring in the body, Oxidation & Reduction

Redox Reactions. Chemical Reactions occurring in the body, Oxidation & Reduction
Recently uploaded
80 ĐỀ THI THỬ TUYỂN SINH TIẾNG ANH VÀO 10 SỞ GD – ĐT THÀNH PHỐ HỒ CHÍ MINH NĂ...

80 ĐỀ THI THỬ TUYỂN SINH TIẾNG ANH VÀO 10 SỞ GD – ĐT THÀNH PHỐ HỒ CHÍ MINH NĂ...Nguyen Thanh Tu Collection
Recently uploaded (20)
Plant propagation: Sexual and Asexual propapagation.pptx

Plant propagation: Sexual and Asexual propapagation.pptx
Basic Civil Engineering first year Notes- Chapter 4 Building.pptx

Basic Civil Engineering first year Notes- Chapter 4 Building.pptx
General Principles of Intellectual Property: Concepts of Intellectual Proper...

General Principles of Intellectual Property: Concepts of Intellectual Proper...
ICT Role in 21st Century Education & its Challenges.pptx

ICT Role in 21st Century Education & its Challenges.pptx
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
Beyond_Borders_Understanding_Anime_and_Manga_Fandom_A_Comprehensive_Audience_...

Beyond_Borders_Understanding_Anime_and_Manga_Fandom_A_Comprehensive_Audience_...
80 ĐỀ THI THỬ TUYỂN SINH TIẾNG ANH VÀO 10 SỞ GD – ĐT THÀNH PHỐ HỒ CHÍ MINH NĂ...

80 ĐỀ THI THỬ TUYỂN SINH TIẾNG ANH VÀO 10 SỞ GD – ĐT THÀNH PHỐ HỒ CHÍ MINH NĂ...
Salient Features of India constitution especially power and functions

Salient Features of India constitution especially power and functions
Unit 3 Emotional Intelligence and Spiritual Intelligence.pdf

Unit 3 Emotional Intelligence and Spiritual Intelligence.pdf
21st_Century_Skills_Framework_Final_Presentation_2.pptx

21st_Century_Skills_Framework_Final_Presentation_2.pptx
Food safety_Challenges food safety laboratories_.pdf

Food safety_Challenges food safety laboratories_.pdf
Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...

Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...
Python Notes for mca i year students osmania university.docx

Python Notes for mca i year students osmania university.docx
Redox o h
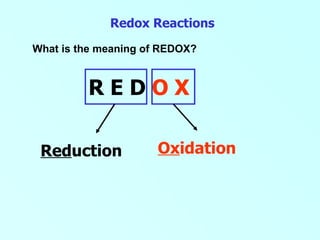
- 1. Redox Reactions What is the meaning of REDOX? R E D O X Ox idation Red uction
- 4. Defn 1 (In terms of oxygen) Oxidation = addition/gain of oxygen A substance that gains oxygen is said to be oxidised. Reduction = removal/loss of oxygen. A substance that loses oxygen is said to be reduced.
- 6. Example based on Defn 1 (In terms of oxygen) 2. Fe 2 O 3 + 3CO -> 2Fe + 3CO 2 Carbon monoxide (CO) is oxidised. Iron (III) oxide (Fe 2 O 3 ) is reduced.
- 7. Defn 2 (In terms of hydrogen) Oxidation = removal/loss of hydrogen A substance that loses hydrogen is said to be oxidised. Reduction = addition/gain of hydrogen. A substance that gains hydrogen is said to be reduced.