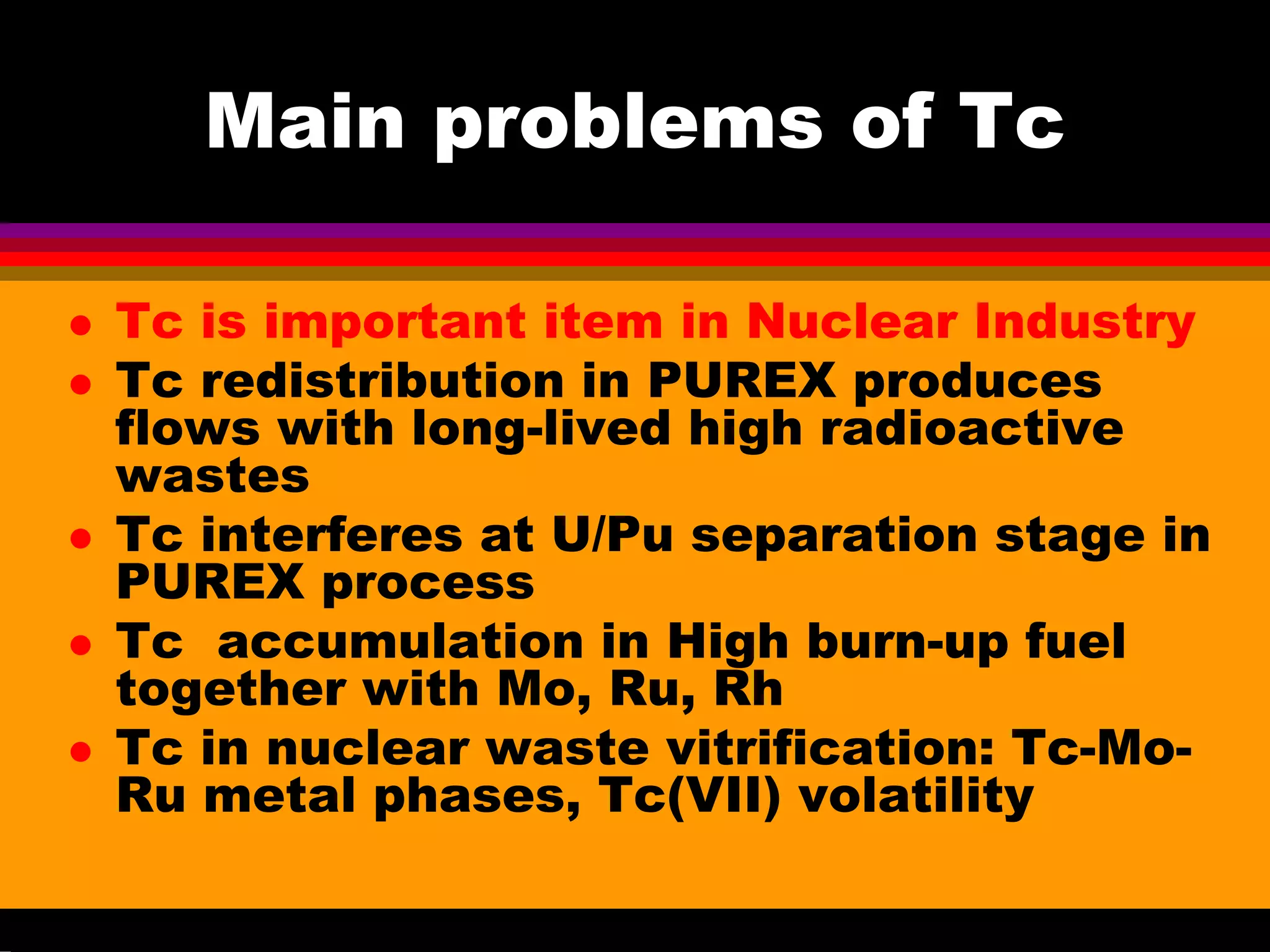
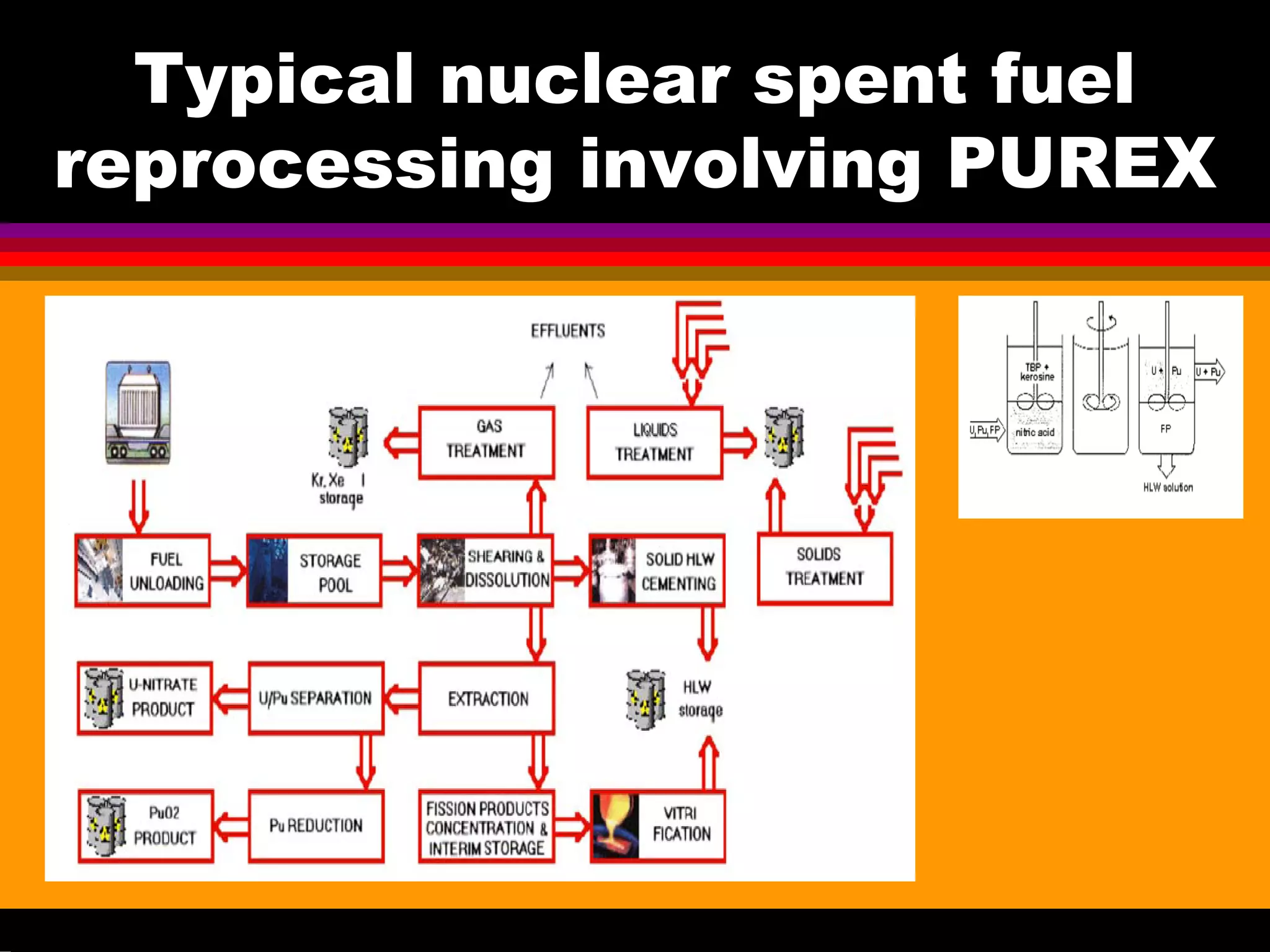
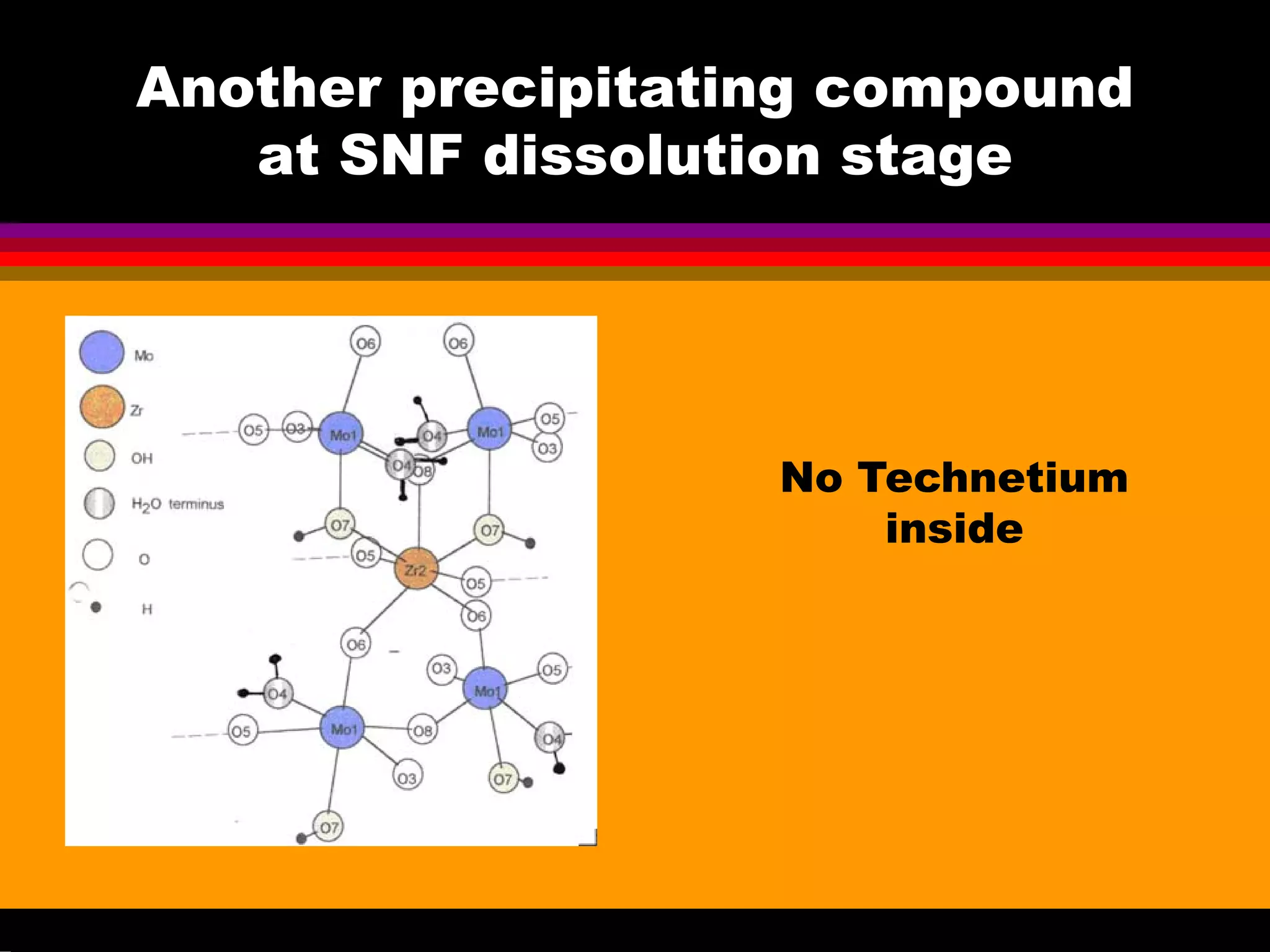
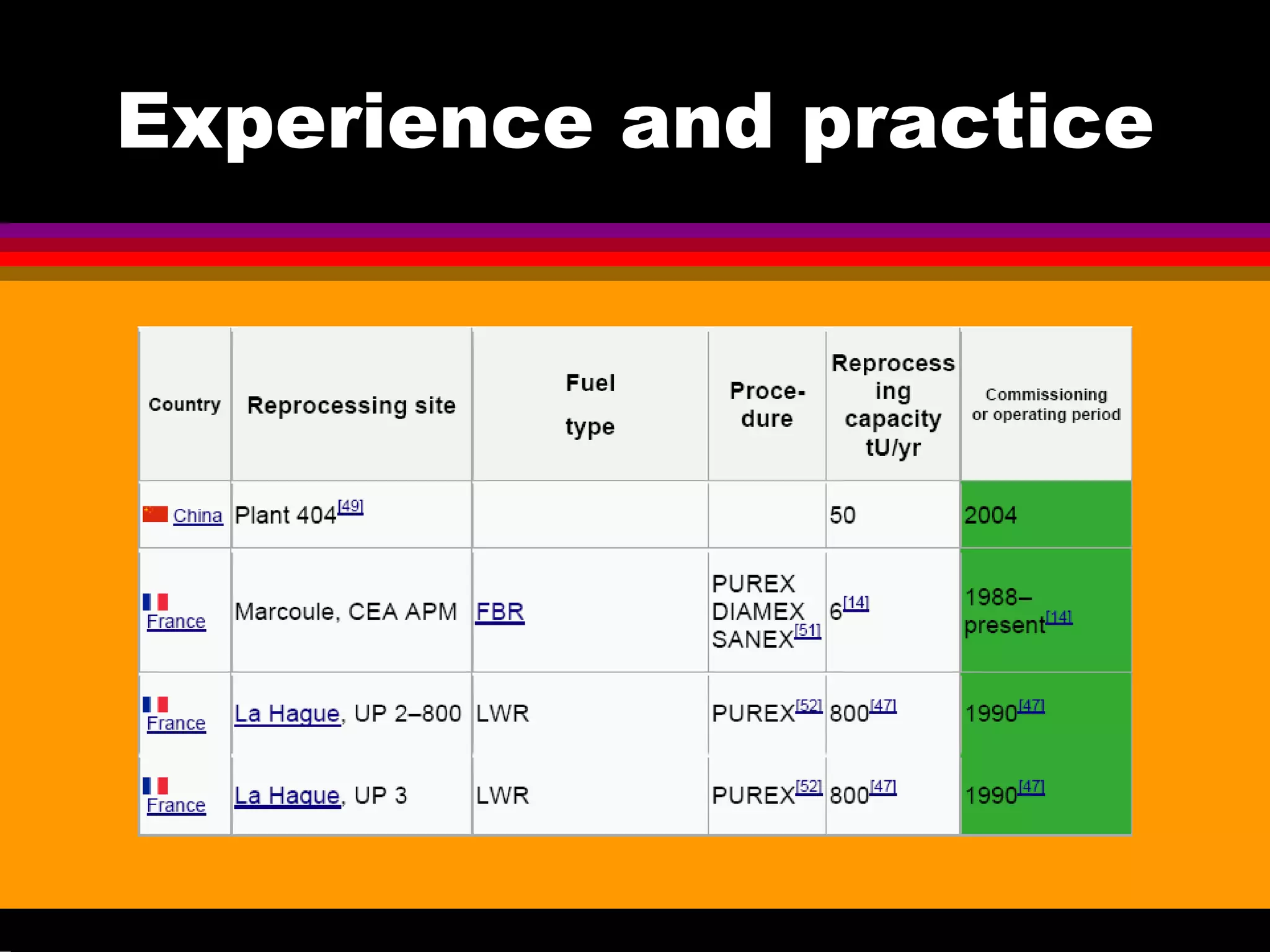
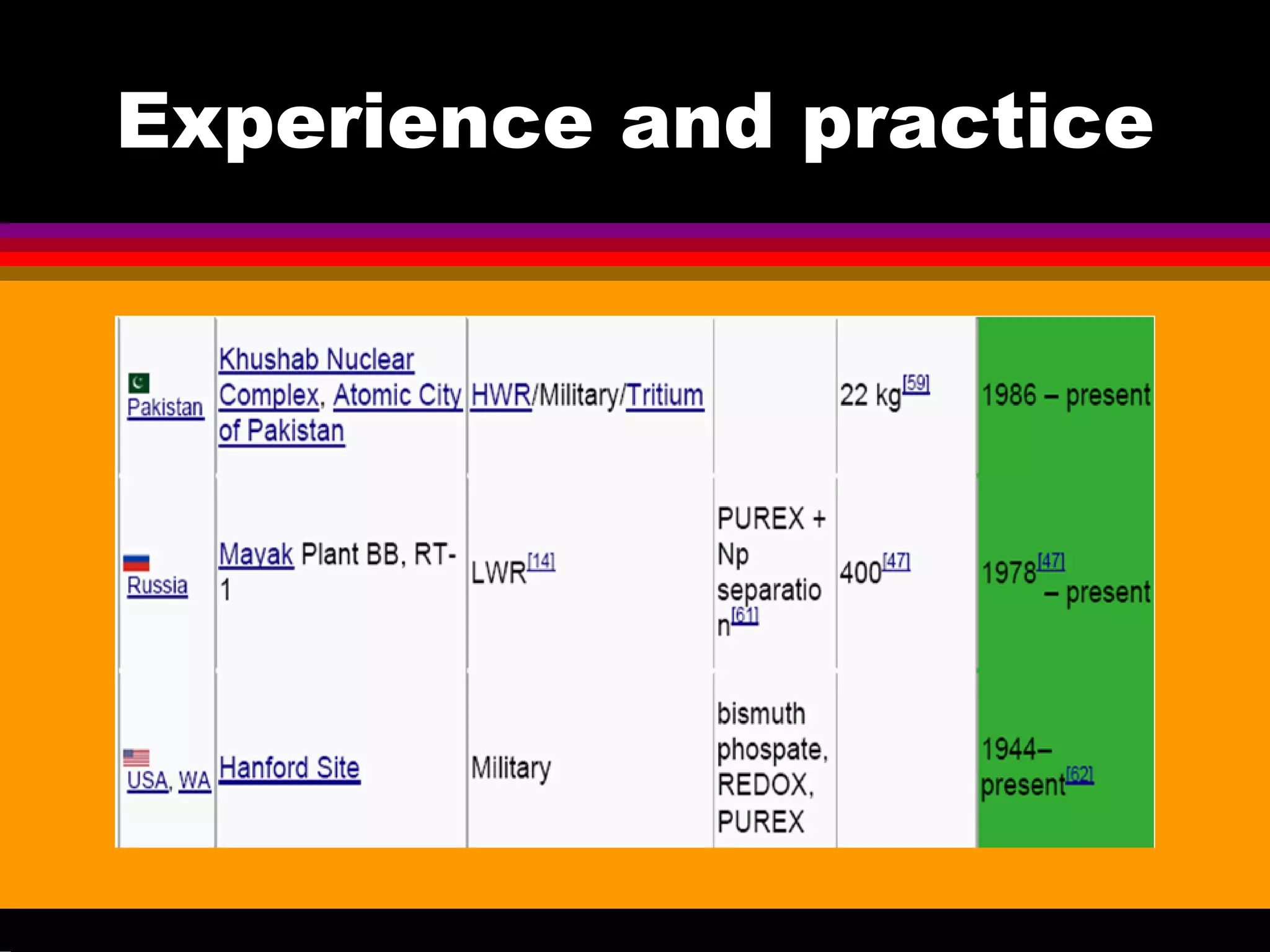
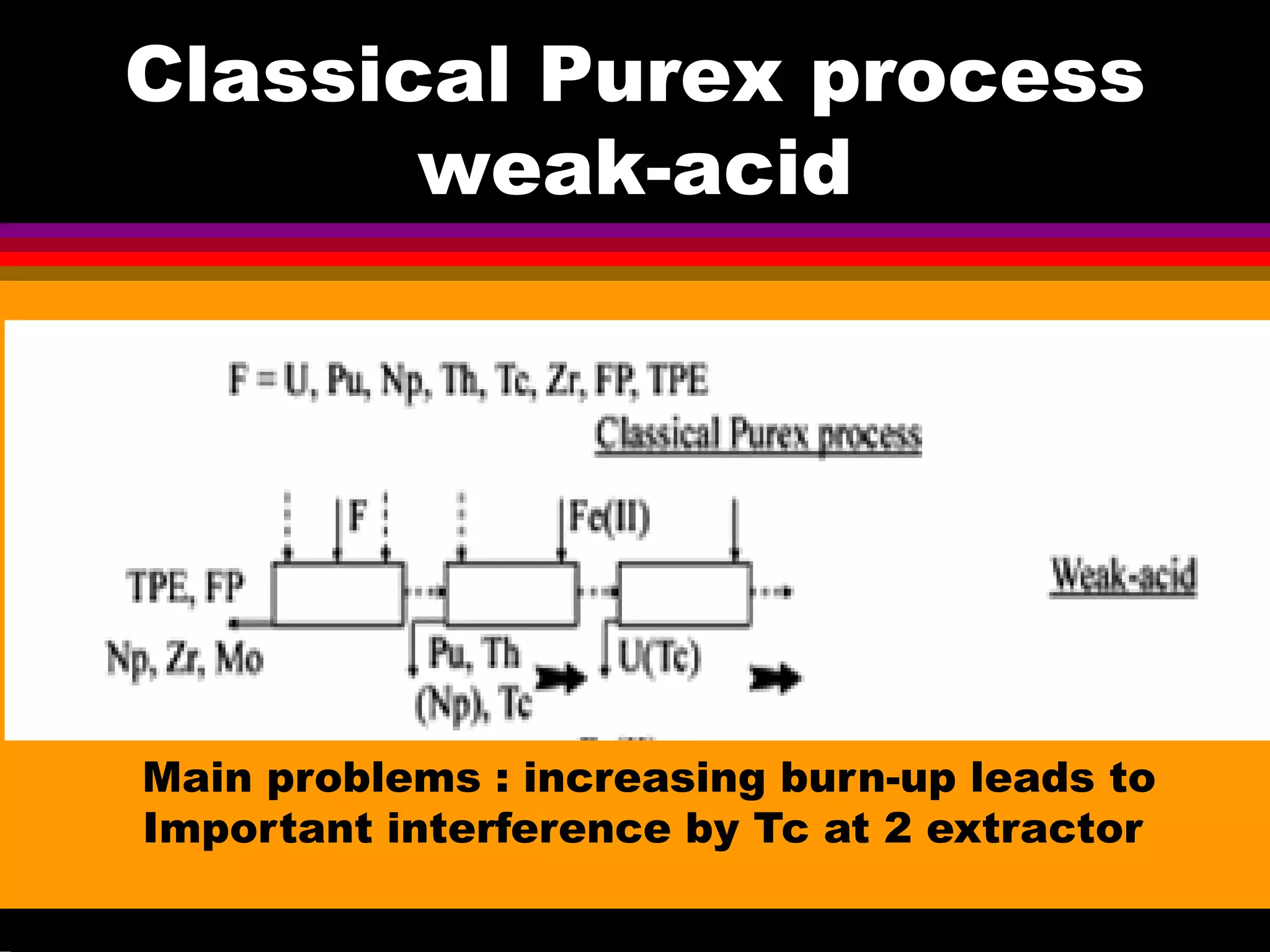
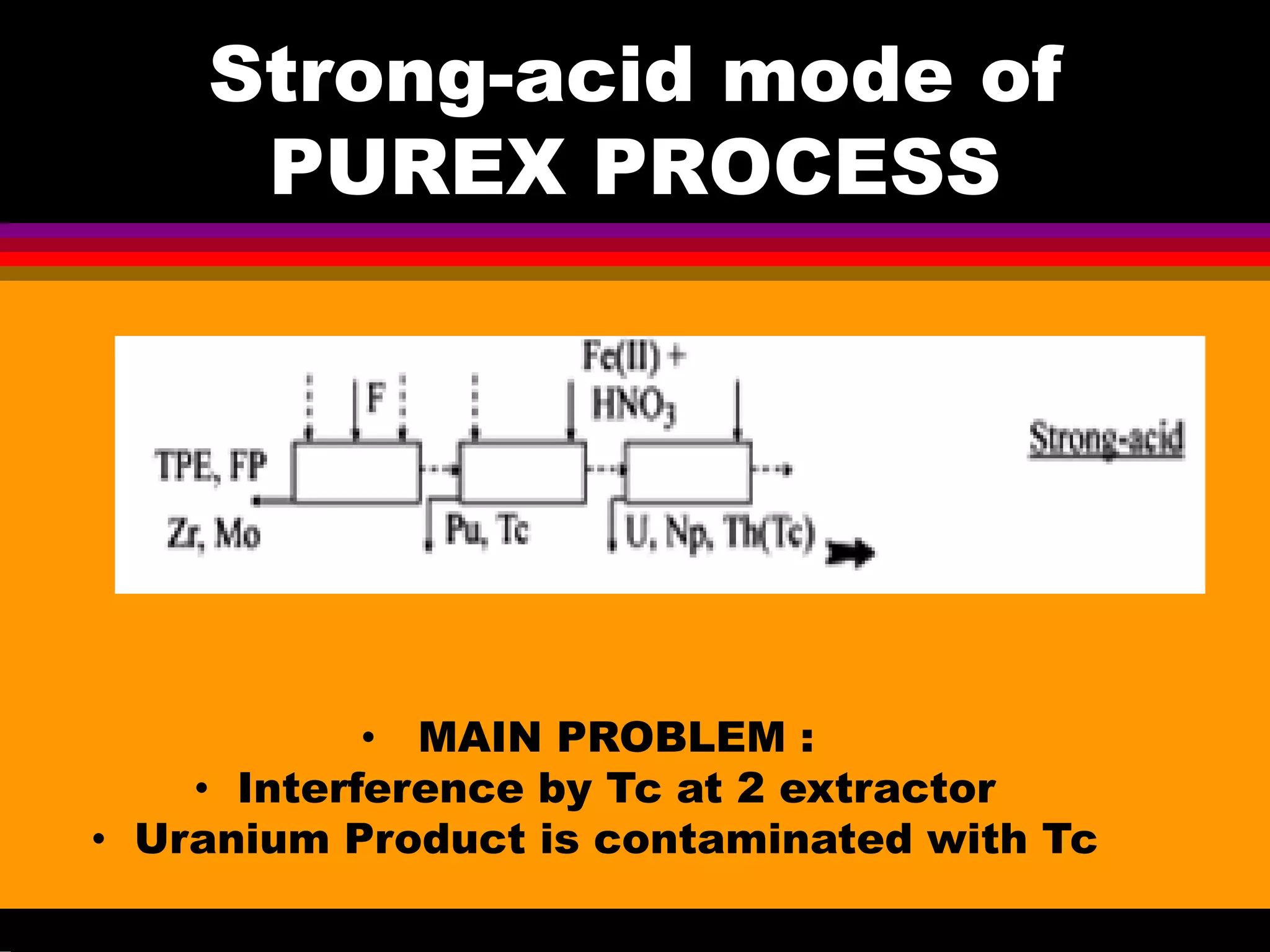
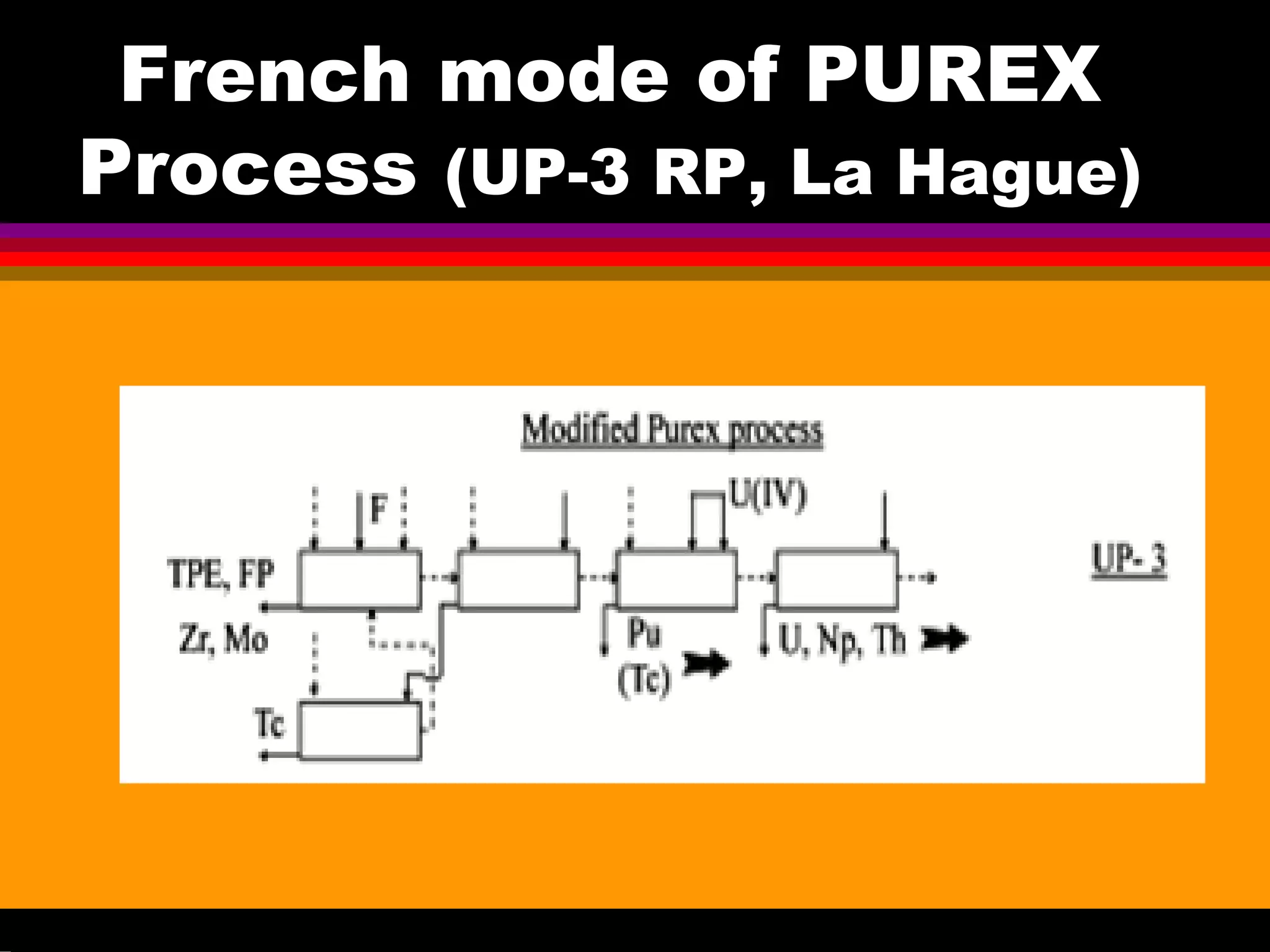
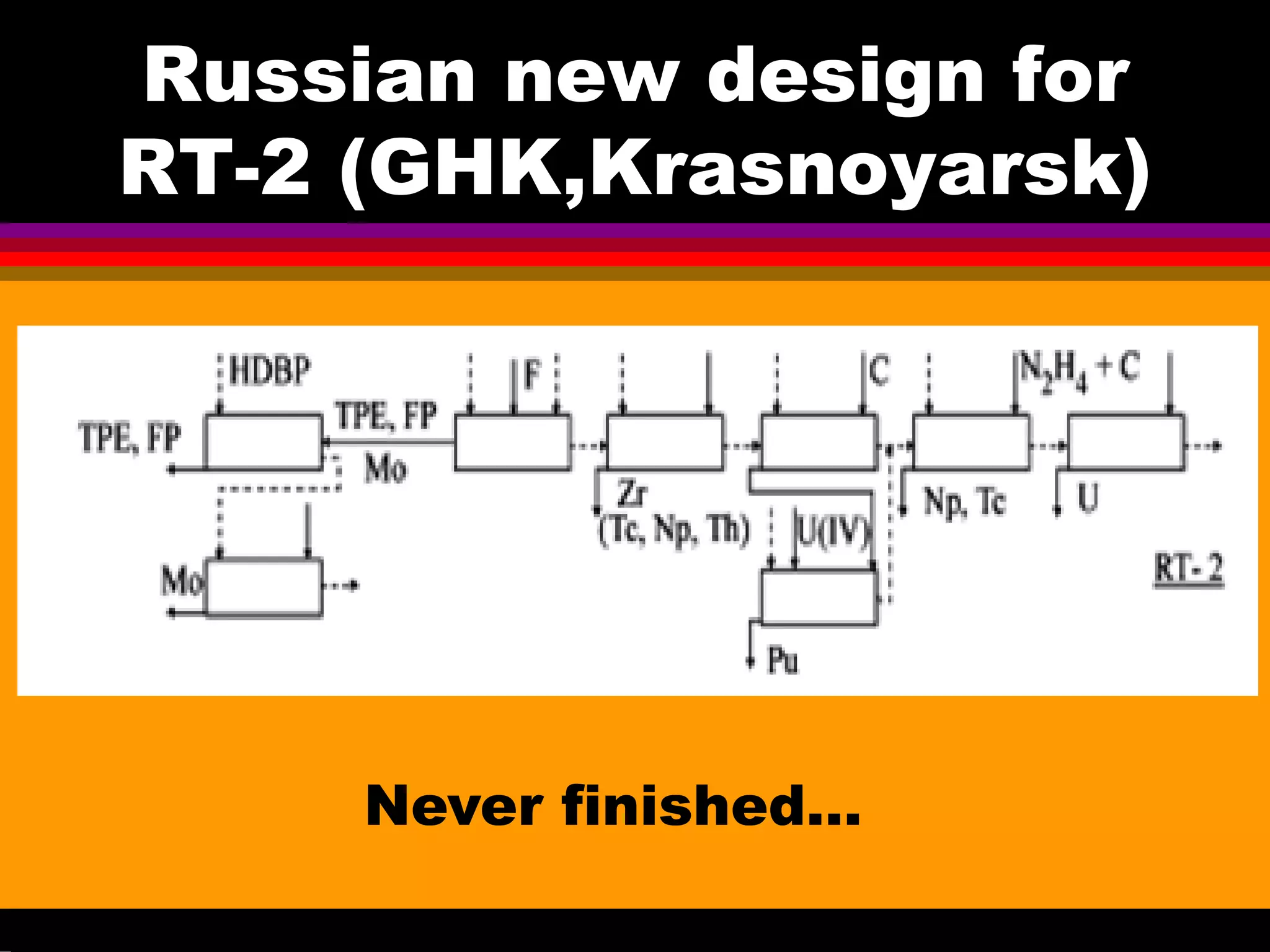
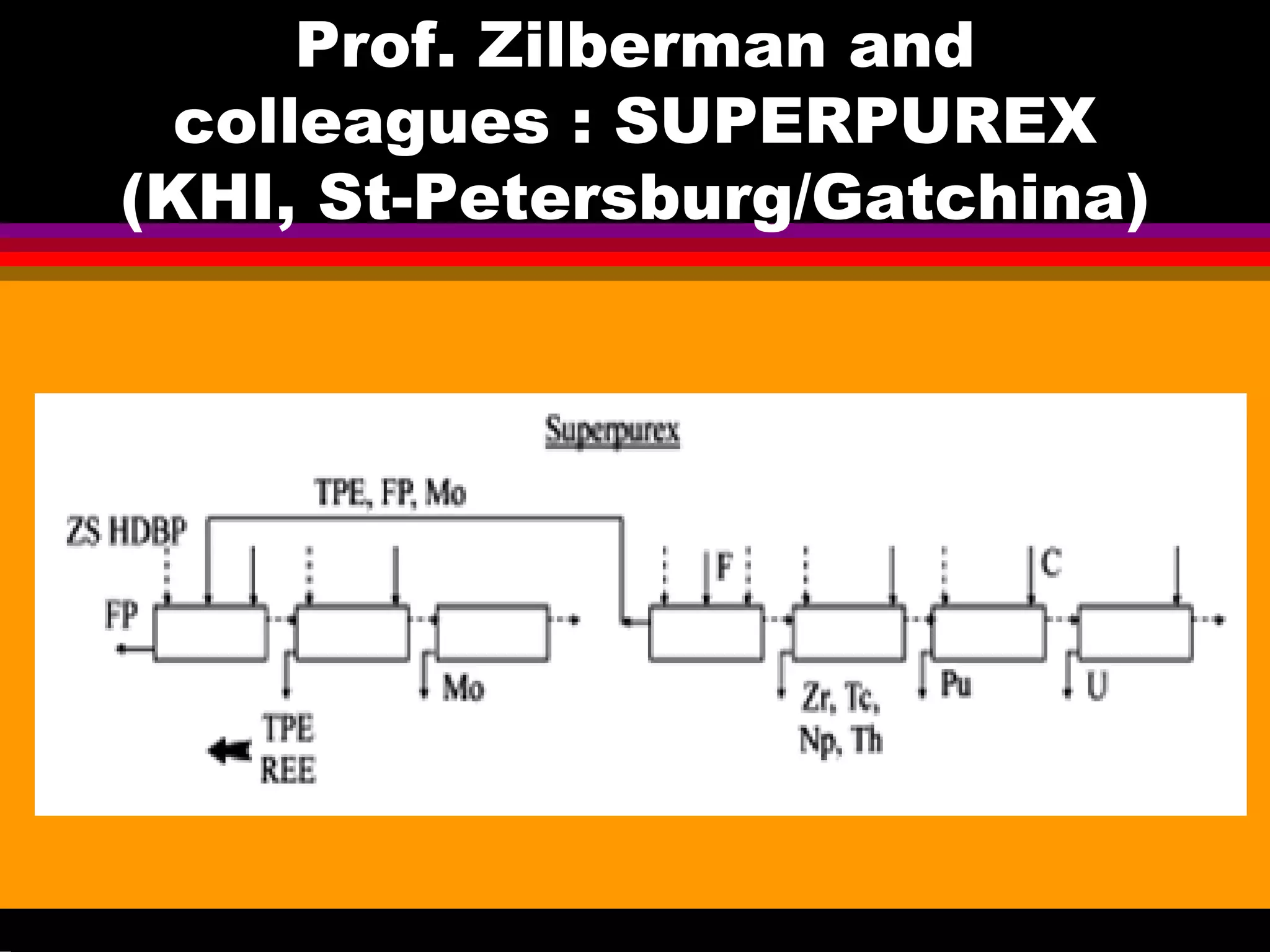
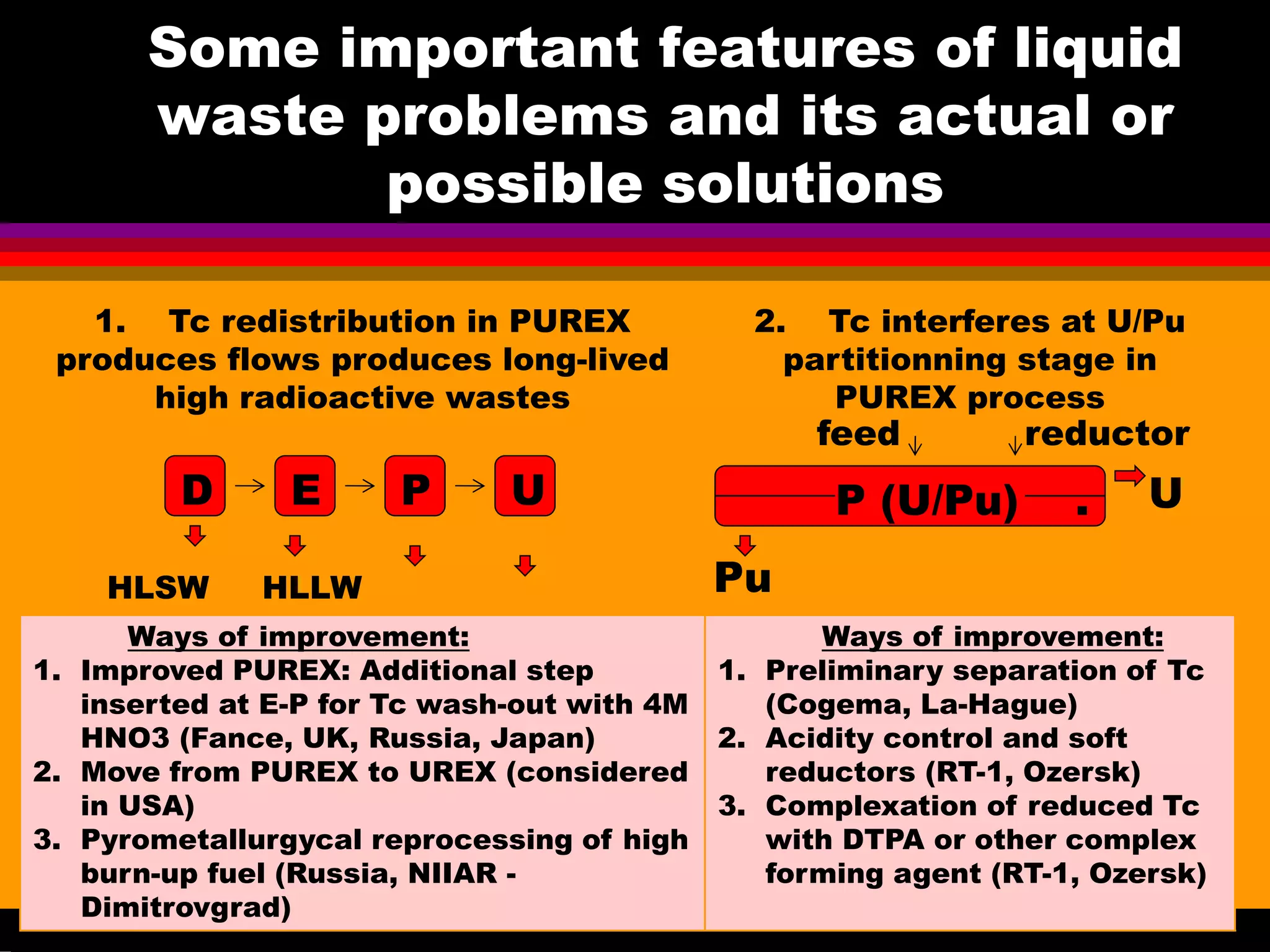
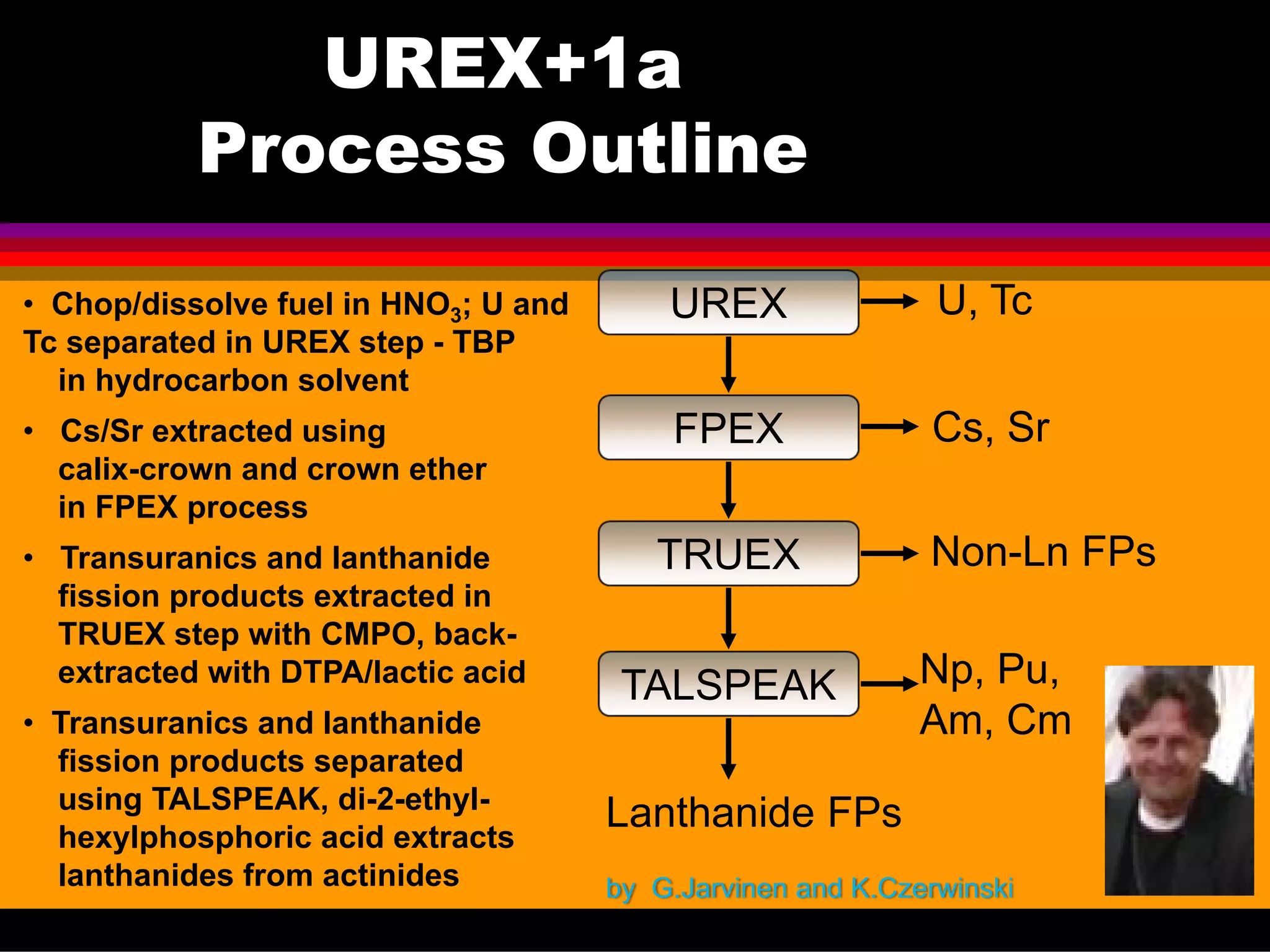
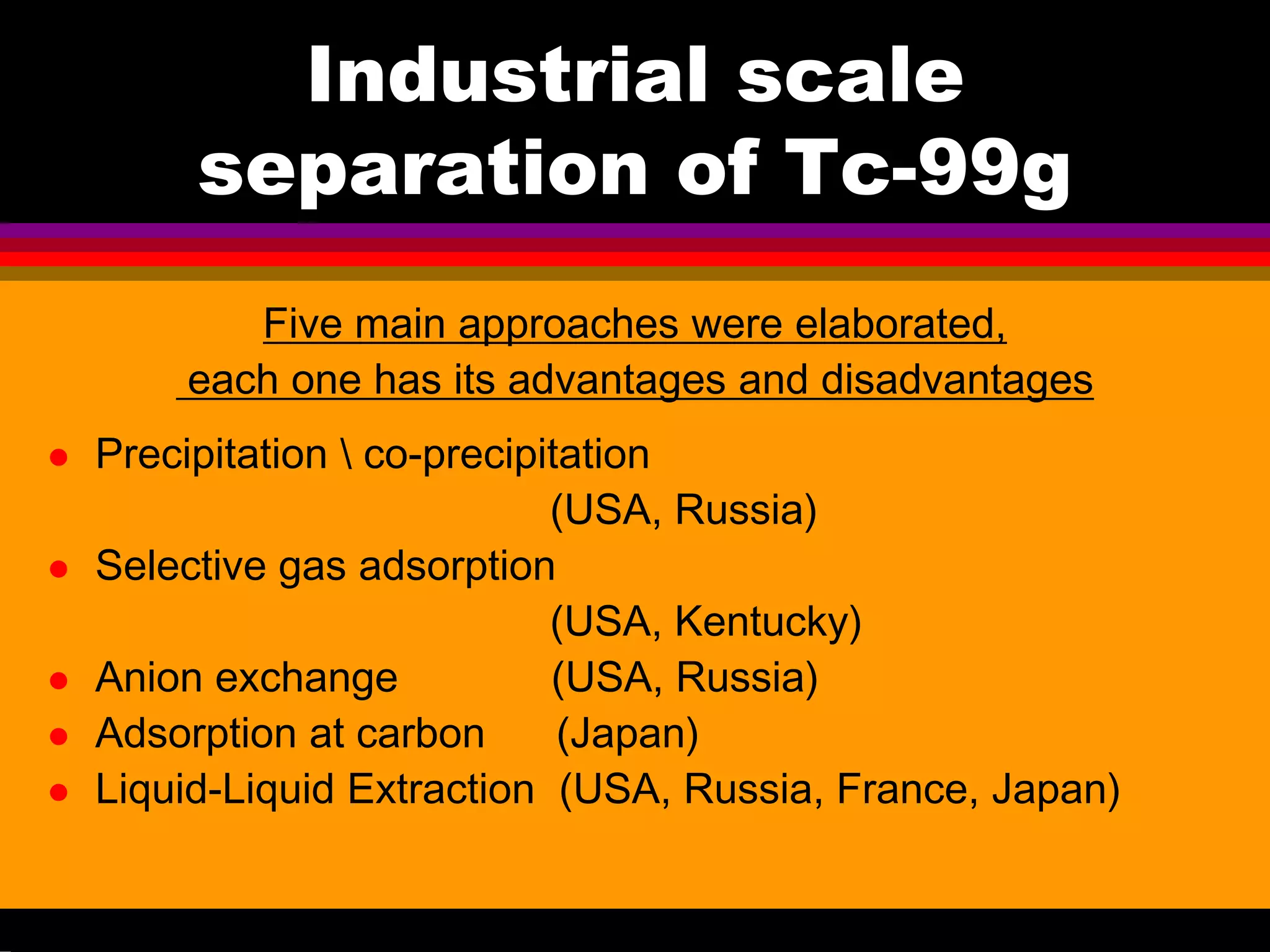
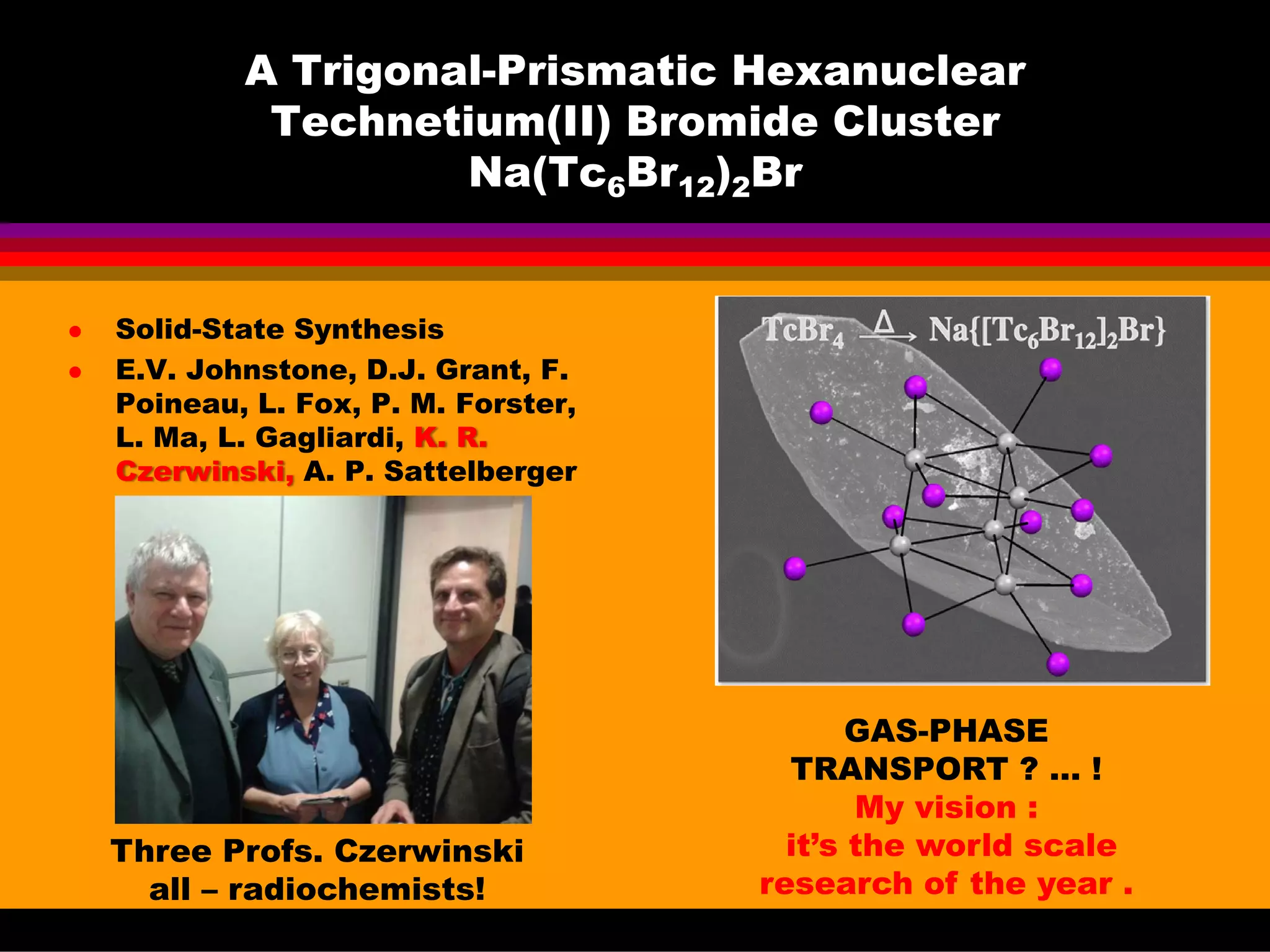
This document discusses technetium separation technologies from spent nuclear fuel. It begins with an overview of technetium discovery and properties. The main challenges of technetium include its interference in uranium/plutonium separation in reprocessing and its accumulation in high-level nuclear waste streams. The document then reviews various separation approaches developed in different countries, including precipitation, gas adsorption, ion exchange, liquid-liquid extraction. It highlights Russian experience with improved PUREX processes and development of ion exchange separation of technetium. The document emphasizes that fundamental studies of technetium chemistry have helped develop better separation methods and manage the challenges of technetium in nuclear applications.




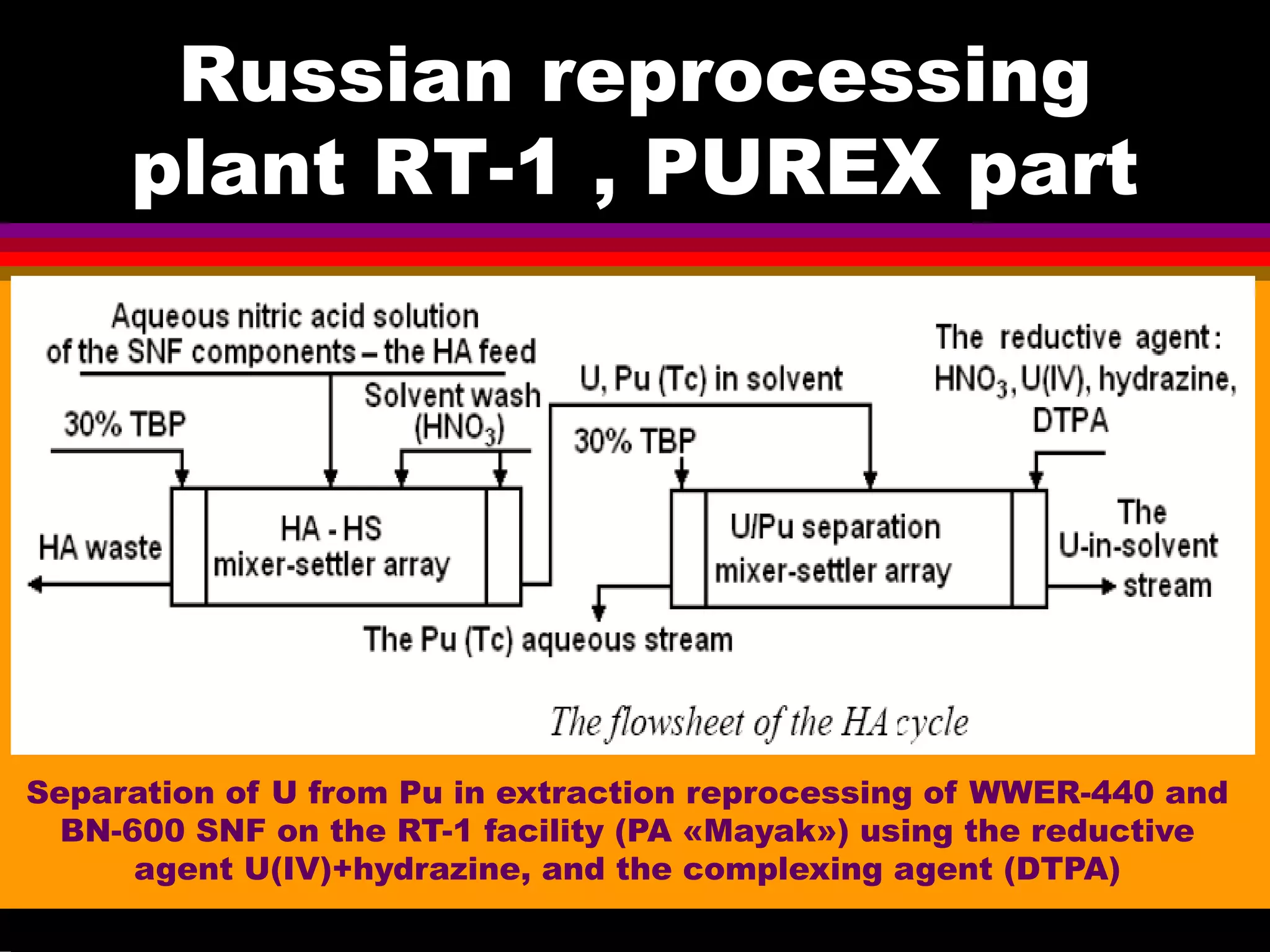
![Some examples of Russian
experience in PUREX
improvement
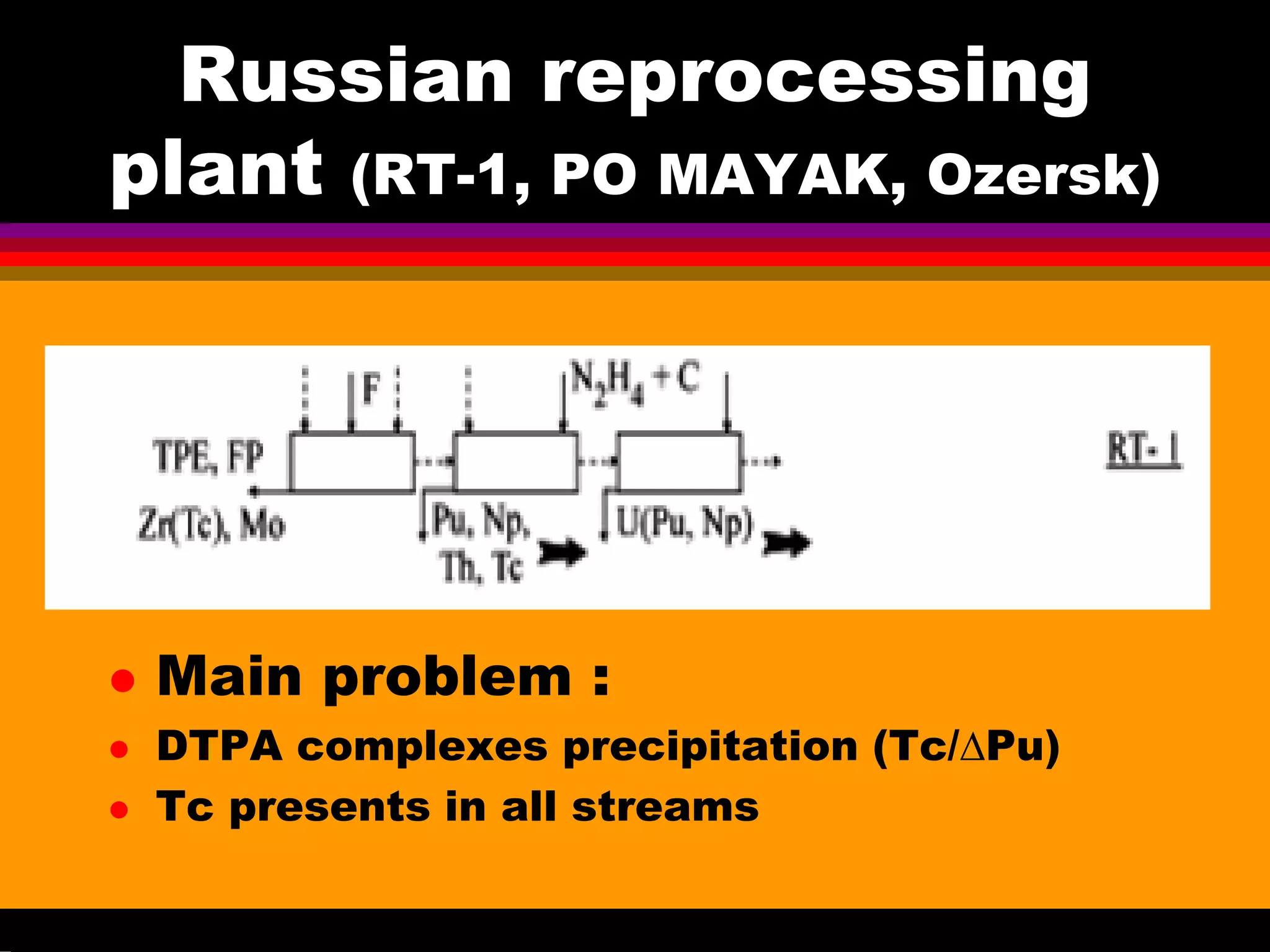
• The first cycle flowsheet of RT-1
plant is essentially similar to the
THORP flowsheet but is
distinguished by more reliable joint
stripping of Pu, Np, and Tc due to
fairly low acidity.
• This is attained owing to
introduction of a special cycle for
separation of Pu and Np using large
amounts of Fe(II);
• As a result, there are serious
problems with evaporation of the
raffinate of Pu-Np purification cyces
and with localization of Tc in the
high-level waste.
•[Zilberman, Radiochemistry 2008]](https://image.slidesharecdn.com/18-09-2013technetiuminreprocessingofspentnuclearfuel-131005102207-phpapp02/75/Technetium-in-reprocessing-of-spent-nuclear-fuel-European-Summer-school-13-2048.jpg)



![Some new Tc(VII) compounds
synthesised in IPCE RAS and
NLVU for reprocessing of SNF
N New compound of Tc or Re Structure C solubility 25°C, M/L ρ Kass
1 Tetrapropylammonium pertechnetate Pna21 a = 13.22(4),
b = 12.35(3),
c = 10.13(4) Å
(8.7 ±0.2) x 10-3 1,26 2,6 ± 0,4
2 Tetrapropylammonium perrhenate Pna21 a = 13.169(2), b =
12.311(2), c =
10.107(1) Å
(8.9 ±0.2) x 10-3 1.57 2,5 ± 0,3
3 Anilinium pertechnetate P21/c 9.8388(2)
5.89920(10) 14.6540(2) Å
(7.9 ± 0.2) x 10-2 2.07 -
4 Anilinium perrhenate P21/c 9.8714(4)
5.9729(2) 14.6354(5)
(8.3 ± 0.2) x 10-2 2.7 -
5 Tetrahexylammonium perthechnetate - (7.1 ± 0.5) x 10-5 1,07 40 ± 5
6 Tetrapentylammonium pertechnetate - (8.0 ± 0.2) x 10-4 1.33 -
7 Threephenylguanidinium pertechnetate P-1 9.87(1) 14.09(1)
15.44(1)
99.6 101.8 95.4
(3.9 ± 0.3) x 10-3 1,3 -
8 LiTcO4*3H2O P63mc, a=7.8604(1)
b=5.4164(1) A
5. 1
9 [(NpO2)2(TcO4)4*3H2O]n
P-1 5.322(5) 13.034(7)
15.46(9)
107.08 98.05 93.86(6)
0.95 4.99](https://image.slidesharecdn.com/18-09-2013technetiuminreprocessingofspentnuclearfuel-131005102207-phpapp02/75/Technetium-in-reprocessing-of-spent-nuclear-fuel-European-Summer-school-35-2048.jpg)
![A few examples of new Tc compound
structures made in IPCE RAS
(K.German, M.Grigoriev, A.Maruk etc.)
[Anil-H]TcO4[GuH]ReO4
LiTcO4*3H2O
[Bu4N]TcO4
[(AnO2)2(MO4)4*3H2O]n ,
(An = U, Np; M = Tc, Re)
[Pr4N]TcO4
[Tc2Ac4](TcO4)2](https://image.slidesharecdn.com/18-09-2013technetiuminreprocessingofspentnuclearfuel-131005102207-phpapp02/75/Technetium-in-reprocessing-of-spent-nuclear-fuel-European-Summer-school-37-2048.jpg)

![Top of the fundamental studies on Tc in IPCE RAS
10 (!) oxidation states were found for
Tc in HX (X = Cl, Br, I) :
7+, 6+, 5+, 4+, 3+, 2.5+, 2+, 1.83+, 1.66+, 1.5+
1. 3-gonal-prismatic Tc chlorides and iodides ( 2 clusters of Tc(1.83+)
and Tc(1.66+) : (Me4N)x[Tc6(m-Cl)6Cl6]Cly ) (K.German and others)
2. 4-gonal-prismatic Tc cluster bromide (addition of Tc2X2 to (1)
S.Kryutchkov)
3. octahedral Tc cluster bromides and iodides (angular conversion of (1))
а
в
1 2 3
Each synthesis involve up to 10 g of Tc !
Structures: unique in inorganic chemistry](https://image.slidesharecdn.com/18-09-2013technetiuminreprocessingofspentnuclearfuel-131005102207-phpapp02/75/Technetium-in-reprocessing-of-spent-nuclear-fuel-European-Summer-school-39-2048.jpg)
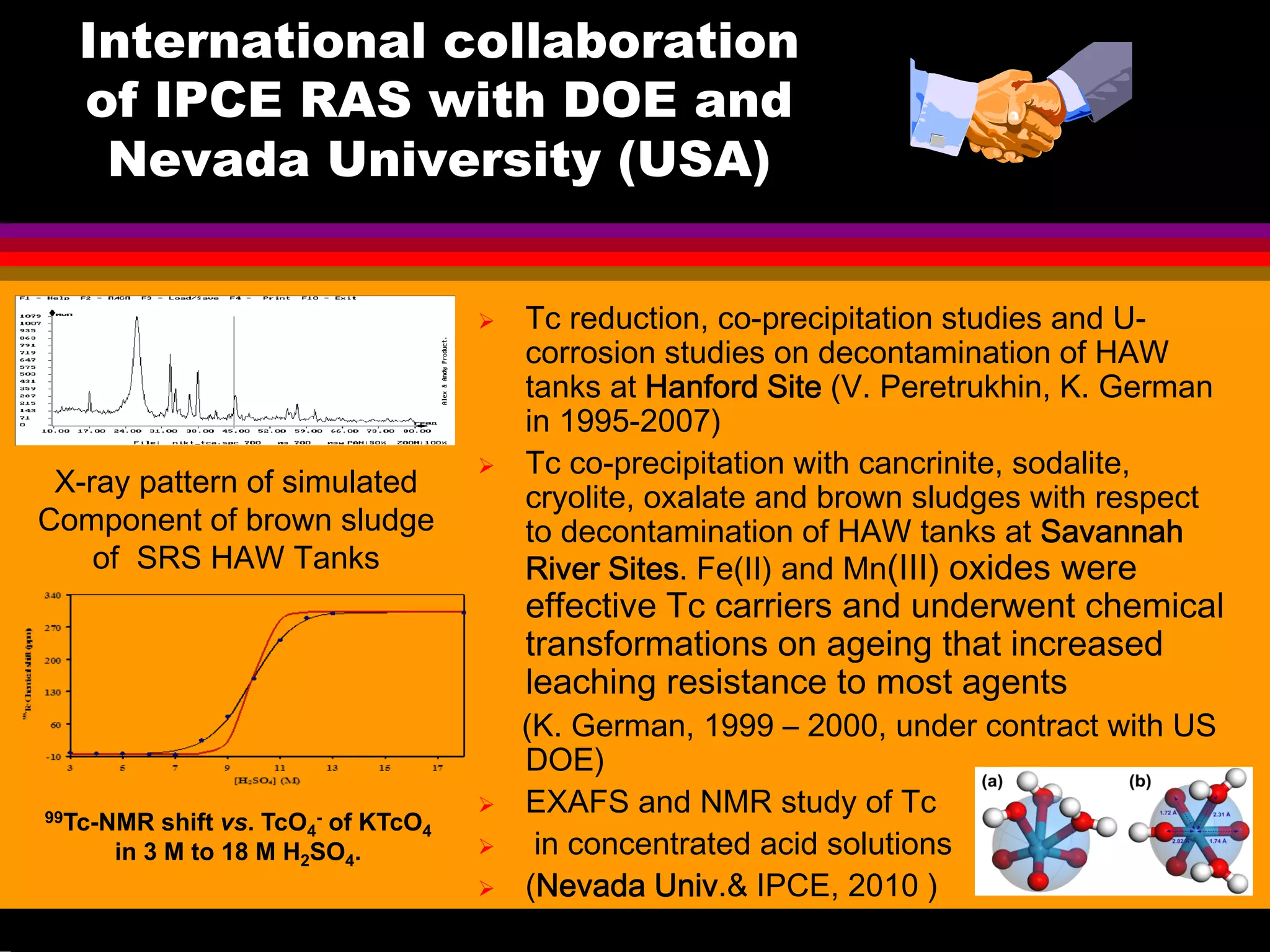
![99Tc concentrations found in
various tank sludges at SRS
Tank
Number
[Tc-99],
mCi/g dried
solids
Reference
17 0.462 d'Entremont et
al. 1997
20, white
solids
0.34 d'Entremont and
Hester 1996
20, brown
solids
0.94 d'Entremont and
Hester 1996
42 0.22 Hay 1999
51 0.21 Hay 1999
8 0.22 Hay 1999
11 0.34 Hay 1999
The discovery of relatively high
99Tc concentrations in
inorganic mineral sludge heels
taken from some tanks at the
US-DOE Savannah River Site
(SRS) has prompted
investigations of Tc uptake
from alkaline highly active
waste (HAW) by solid
adsorbents](https://image.slidesharecdn.com/18-09-2013technetiuminreprocessingofspentnuclearfuel-131005102207-phpapp02/75/Technetium-in-reprocessing-of-spent-nuclear-fuel-European-Summer-school-43-2048.jpg)
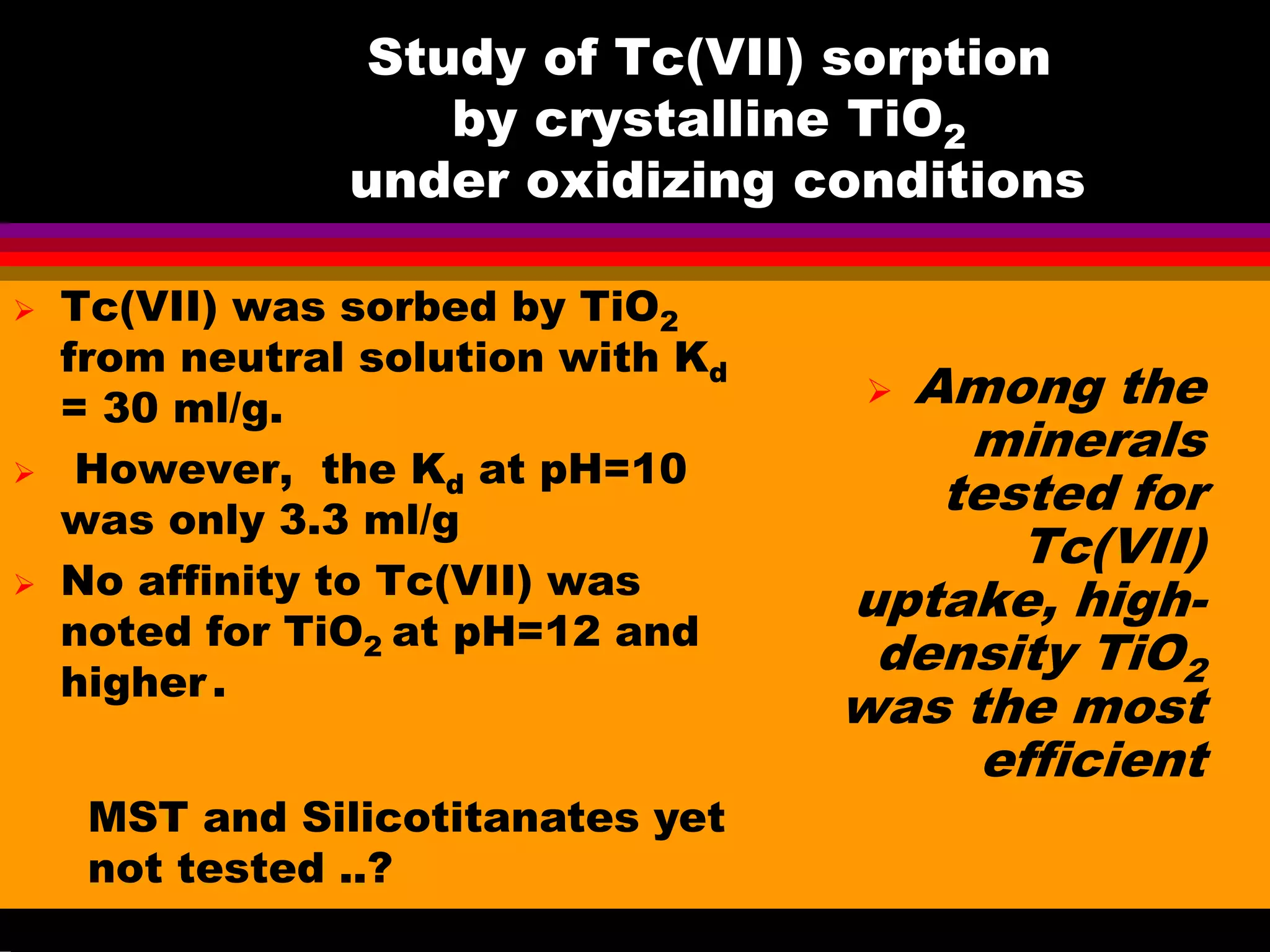
![The SRS waste volumes (Table 2.4 of "Integrated
Database Report - 1993: S.Spent Fuel and Radioactive
Waste Inventories, Projections, and Characteristics,”]
Tc-99 quantities (Table 2.11), and
Volume, Tc-99, Ci [Tc-99], [Tc], 106 Kd
liters Ci/liter g/liter total
Liquid 61.4 1.68E+04 2.74E-03 0.162 -
Sludge 13.9 1.14E+04 8.20E-03 0.483 3
Salt Cake 53.8 2.78E+03 5.17E-04 0.0305 0.2
Overall waste 129.1 3.098E+04 2.40E-03 0.141 -
Question was: Which components absorb Tc with Kd
higher than 3 and are resistant to leaching?
Tc-99 concentrations
calculated from these data](https://image.slidesharecdn.com/18-09-2013technetiuminreprocessingofspentnuclearfuel-131005102207-phpapp02/75/Technetium-in-reprocessing-of-spent-nuclear-fuel-European-Summer-school-44-2048.jpg)
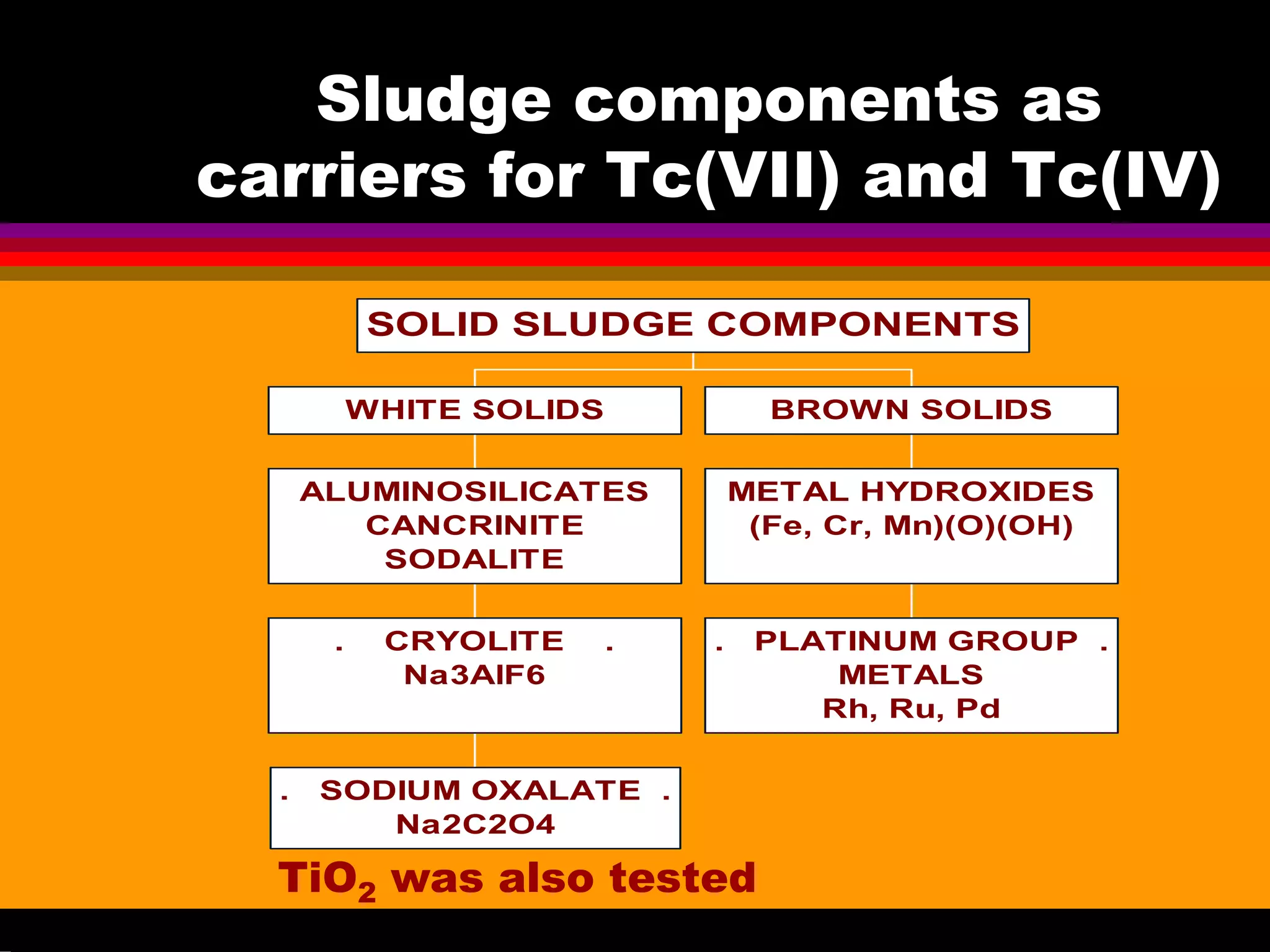
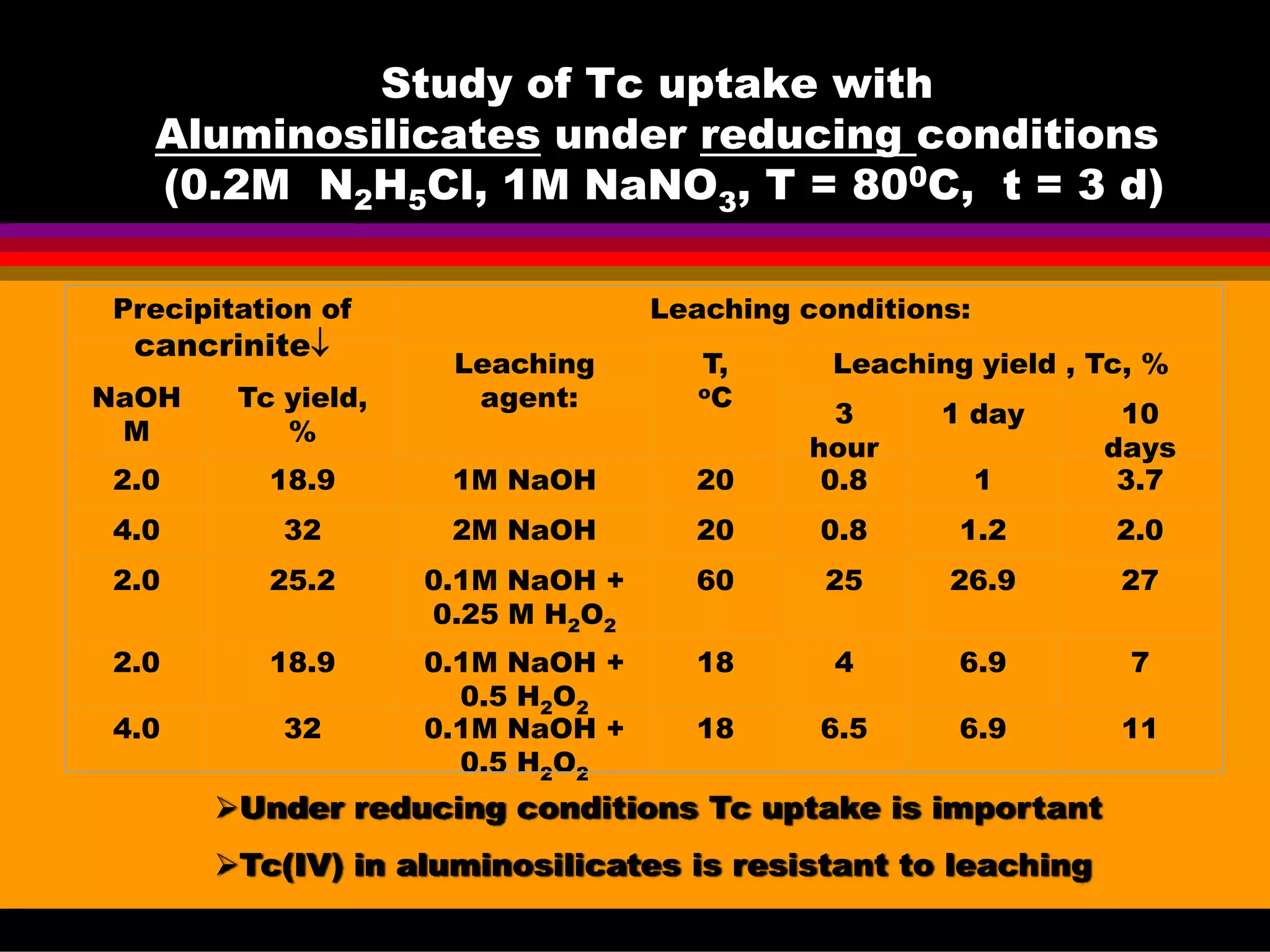
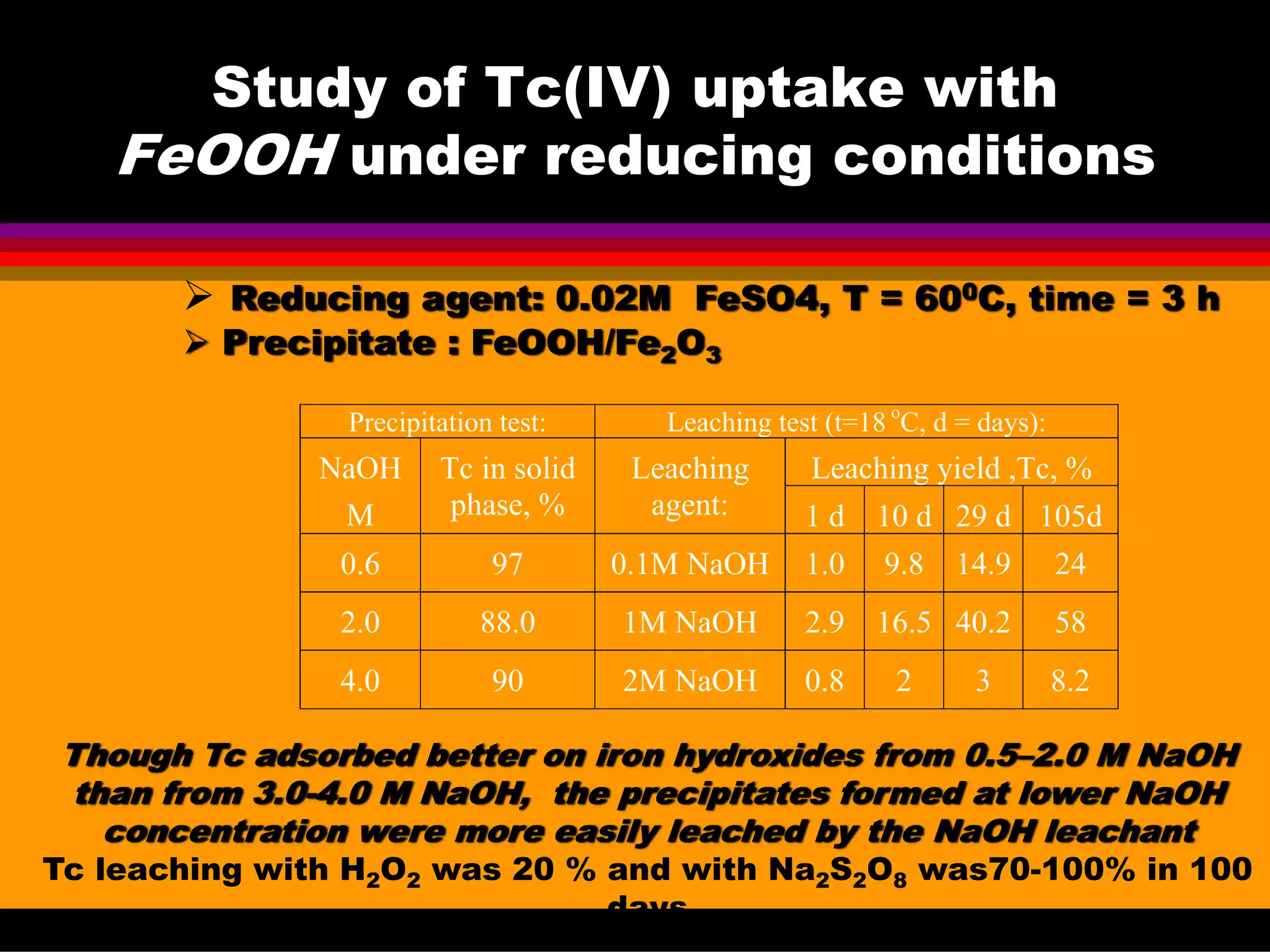
![Experimental conditions for
precipitation and leaching tests:
Precipitation tests:
Wastes are alkaline
Tc is redox sensitive
Sharp differences in the
redox potential within the
tanks are observed,
So, both:
oxidizing [Tc(VII)]
and reducing [Tc(IV)]
conditions were tested in
0.1- 5 N NaOH + 0-5 N NaOH.
Leaching modes:
Surface leaching.
Complete dissolution.
Leaching agents
all precipitates : 0.1N NaOH
aluminosilicates - NaHF2
Na oxalate - 0.1N NaOH, NaNO2
FeOOH - 0.1N NaOH, H2O2
MnOOH - 0.1N NaOH, H2O2
TiO2 - 0.1- 3N NaOH
Methods: Liquid scintillation counting (LSC) of solutions, XRD, NMR, IR](https://image.slidesharecdn.com/18-09-2013technetiuminreprocessingofspentnuclearfuel-131005102207-phpapp02/75/Technetium-in-reprocessing-of-spent-nuclear-fuel-European-Summer-school-46-2048.jpg)
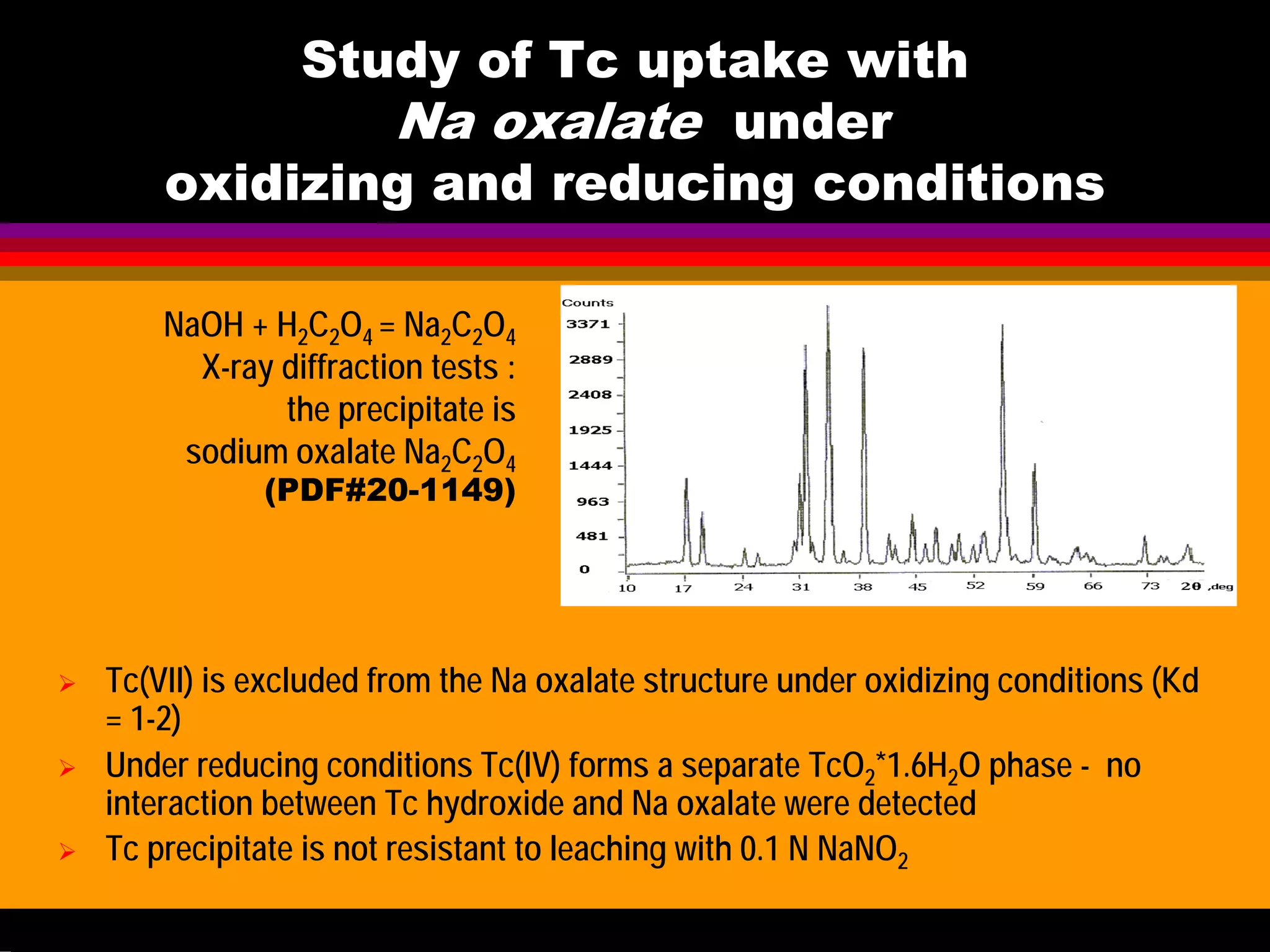
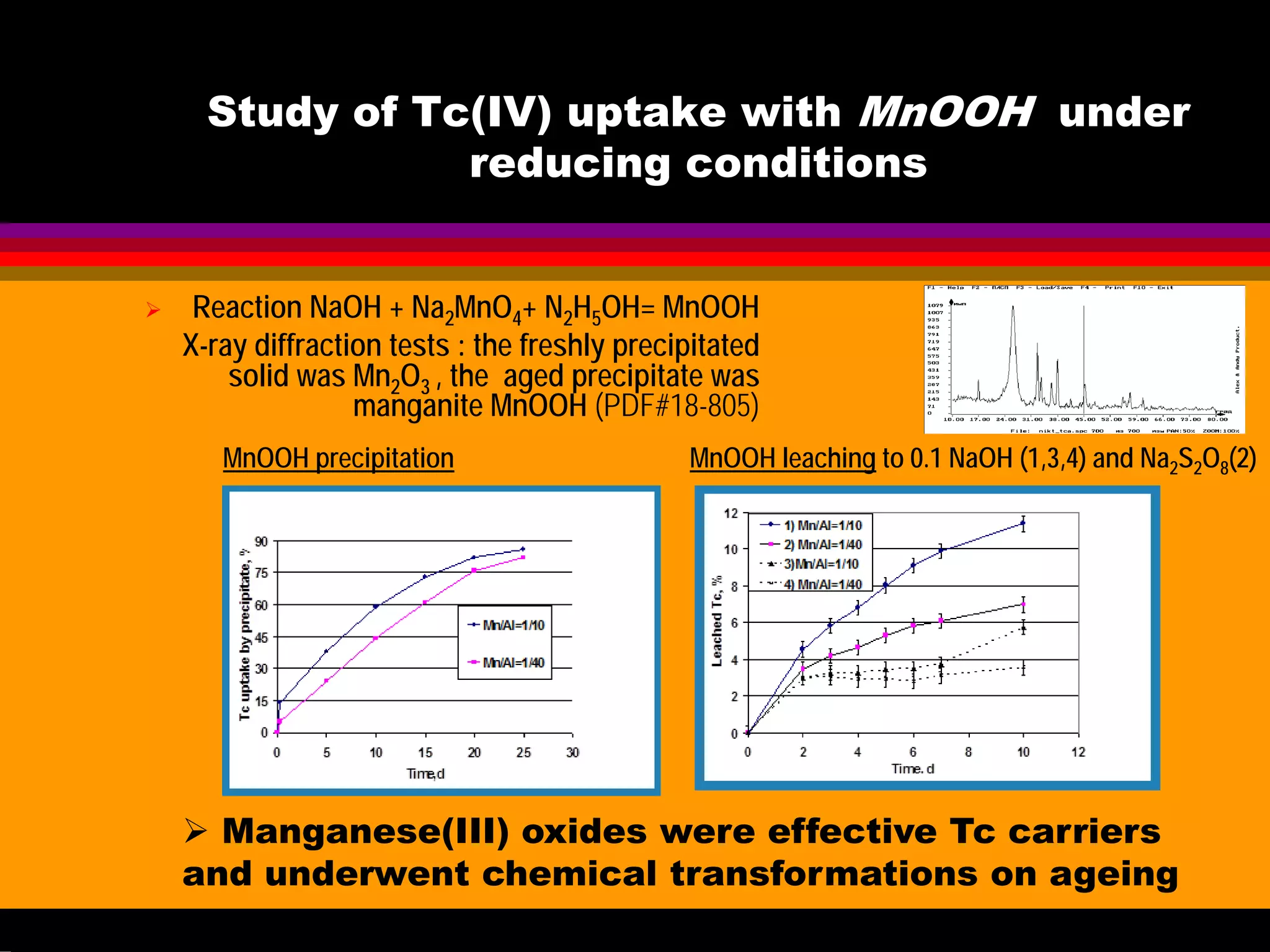
![Study of Tc uptake with
Aluminosilicates under oxidizing
conditions at 70-130oC
Solution Formed solid Kd
10-3
-10-5
M Tc
0.2-5M NaOH
0.5-5 M NaNO3
Cancrinite less 1
10-3
-10-5
M Tc
0.2-5M NaOH
NaNO3 free
Sodalite less 1
TcO4
- is too large
and therefore it is
excluded from the
aluminosilicate
structure in both
cancrinite and
sodalite
Literature data have demonstrated the
possibility of ClO4
- and MnO4
- co-crystallisaton
with aluminosilicates : purple
Na8[AlSiO4]6(MnO4)2 (Weller,1999 etc.)
OUR EXPERIMENTS on TcO4
- (reaction: NaAlO2+Na2SiO3+NaOH)](https://image.slidesharecdn.com/18-09-2013technetiuminreprocessingofspentnuclearfuel-131005102207-phpapp02/75/Technetium-in-reprocessing-of-spent-nuclear-fuel-European-Summer-school-47-2048.jpg)
![Case of Aluminosilicates formed in
concentrated Tc(VII) solution
[Tc] = 0.2 M
in NaNO3 solutions -
cancrinite
in NaNO3-free solutions -
sodalite
Although NMR spectrum
presented shift typical for
coordinated Tc(VII) its
concentration is very low
Dissolution in NaHF2 and LSC
has shown : [Tc] in solid
cancrinite was 57 mg/kg ~ 100
times less than in initial
solution
Fig. 1. NMR-99
Tc spectrum of the aluminosilicate containing
57 mg-Tc/kg. Tc spectrum presents evidence for -30 ppm shift
characteristic of coordinated pertechnetate](https://image.slidesharecdn.com/18-09-2013technetiuminreprocessingofspentnuclearfuel-131005102207-phpapp02/75/Technetium-in-reprocessing-of-spent-nuclear-fuel-European-Summer-school-48-2048.jpg)
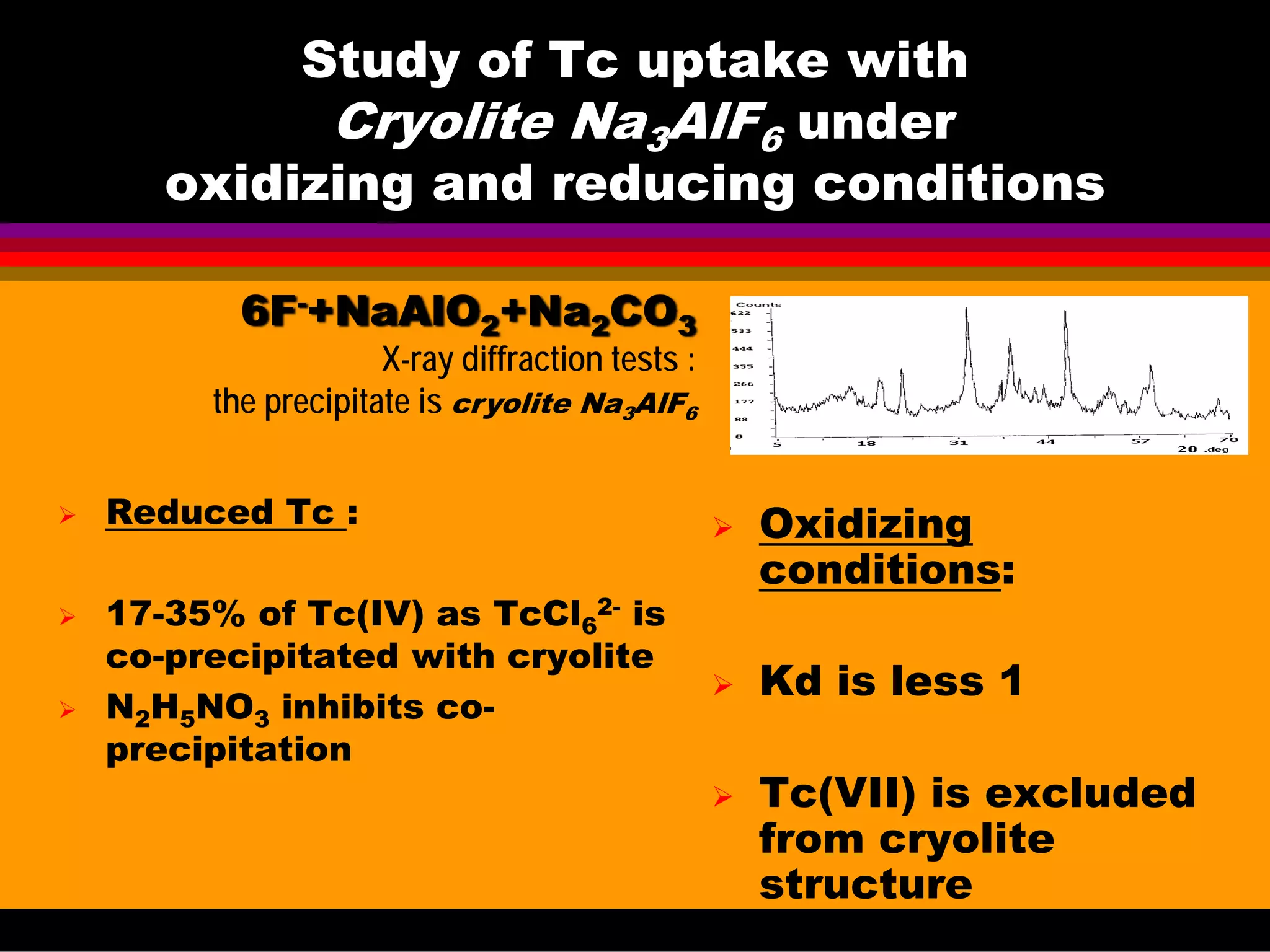
![Tc(IV) uptake with Cryolite Na3AlF6
under reducing conditions
N
o
[NH4F]
initial,
M
[Na2CO3] in
final
solution, M
[N2H5NO3],
in final
solution, M
Tc(IV)
uptake,
%
1
2
3
4
5
8
9
10
2,0
2.5
3.0
4,0
6,0
2,0
2,0
2,0
0,6
0.6
0,6
0.6
0,6
0,4
0,8
0,6
-
-
-
-
-
-
-
0,1
20
23
26
28
35
25
17
0
• Tc(IV) is added as Na2TcCl6 to (NH4F+NaAlO2) solution
• No additional reducing agent in exp. No 1-9
• Leaching test were impossible to quantify relative to real cryolite
in tanks as complete peptization occurred.](https://image.slidesharecdn.com/18-09-2013technetiuminreprocessingofspentnuclearfuel-131005102207-phpapp02/75/Technetium-in-reprocessing-of-spent-nuclear-fuel-European-Summer-school-53-2048.jpg)
![The IPCE publications
used in the presentation
The principle achievements of recent Russian researches in technetium chemistry, metallurgy, environmental science, nuclear
reprocessing and applications are overviewed. The allied aspects of rhenium chemistry and applications are compared. The
progress in technetium handling during the spent nuclear fuel reprocessing was based on the fundamental studies of numerous
new technetium mono- and polynuclear compounds and species [1-10]. The previous achievements were reviewed in [11].
In concentrated water solutions Tc(VII) often forms crystals isomorphous with perchlorates while in concentrated unhydrous
solutions Tc(VII) behaviour is more similar to Re(VII) compared to Cl(VII) [4-6].
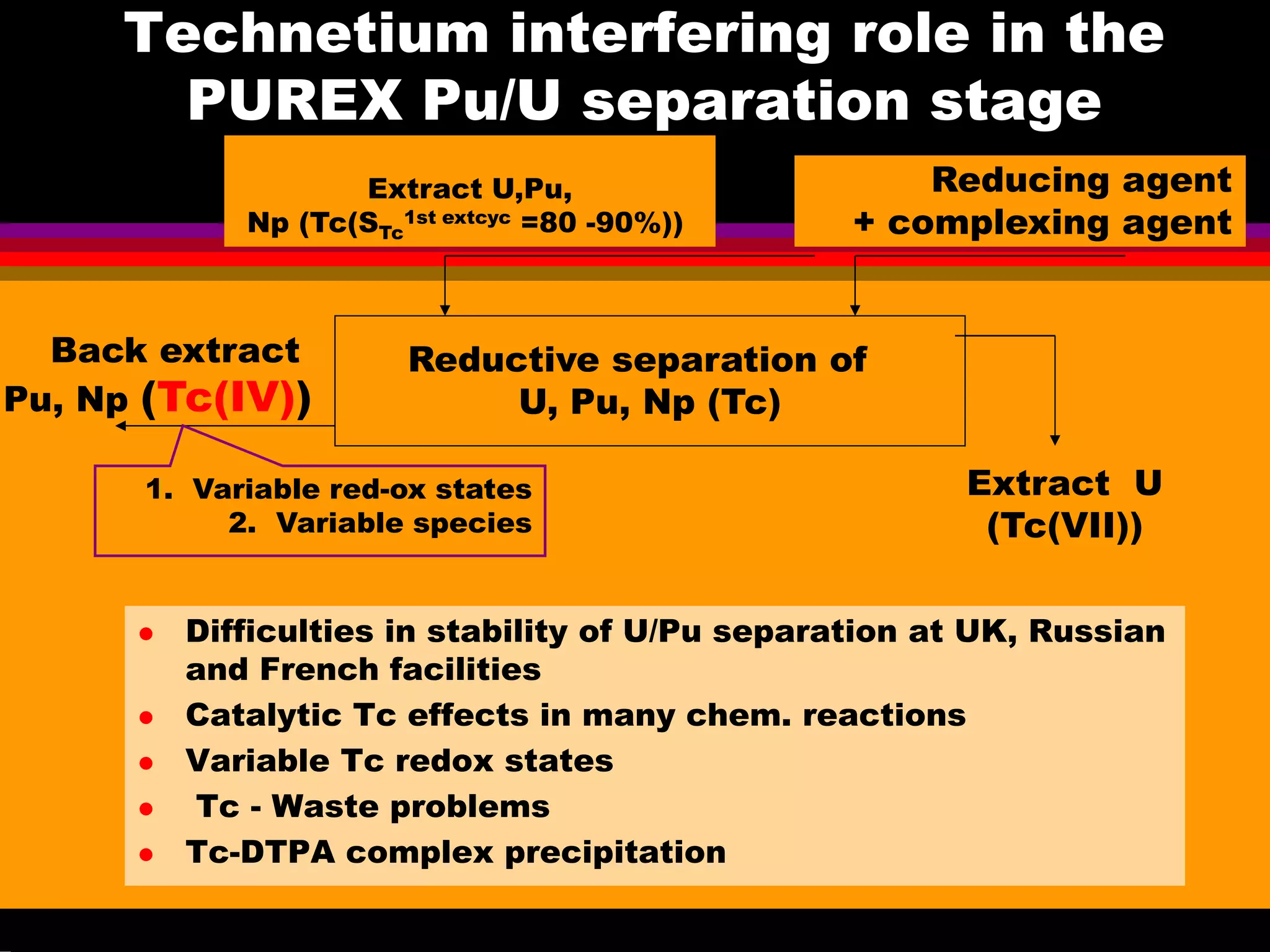
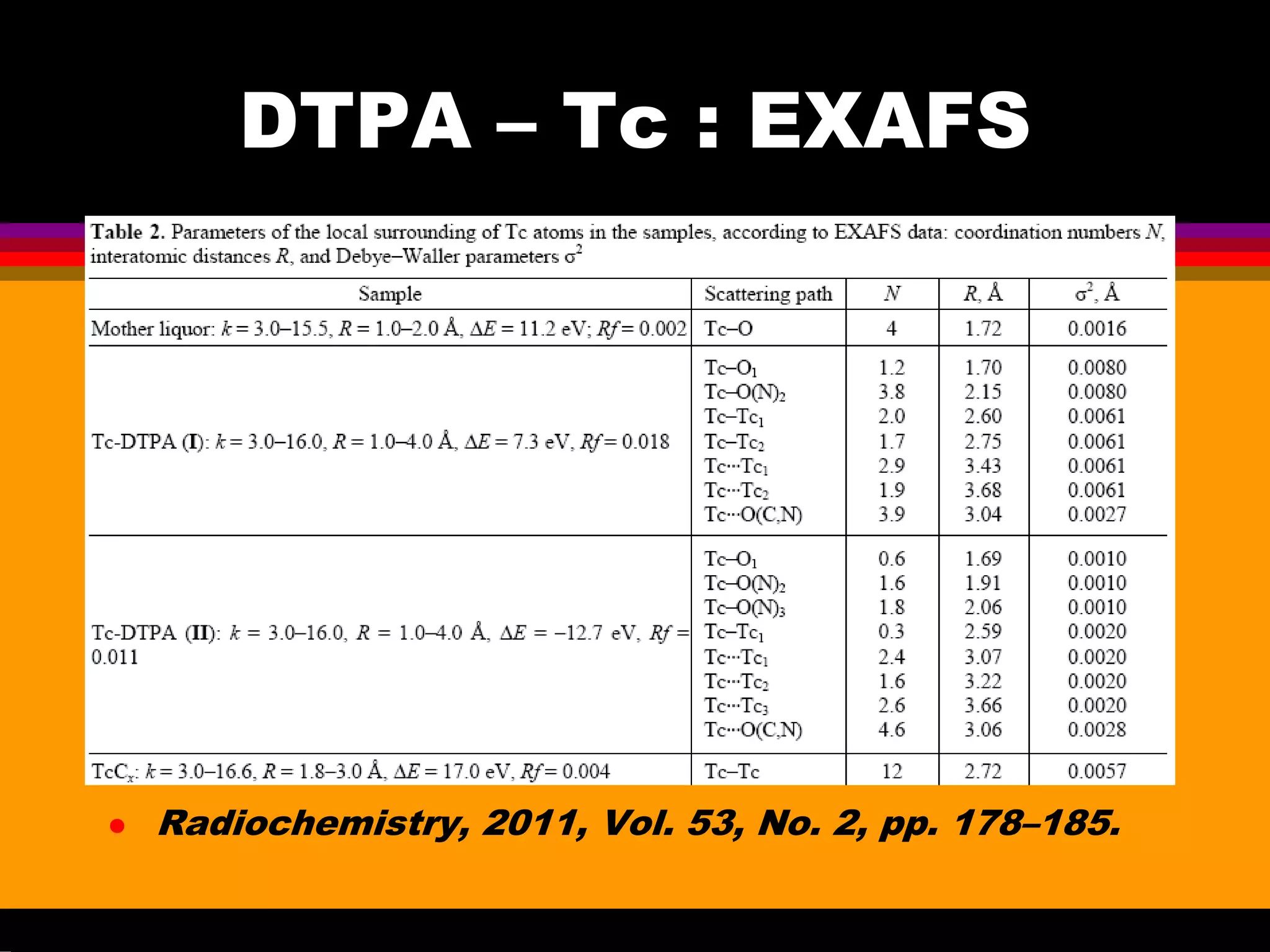
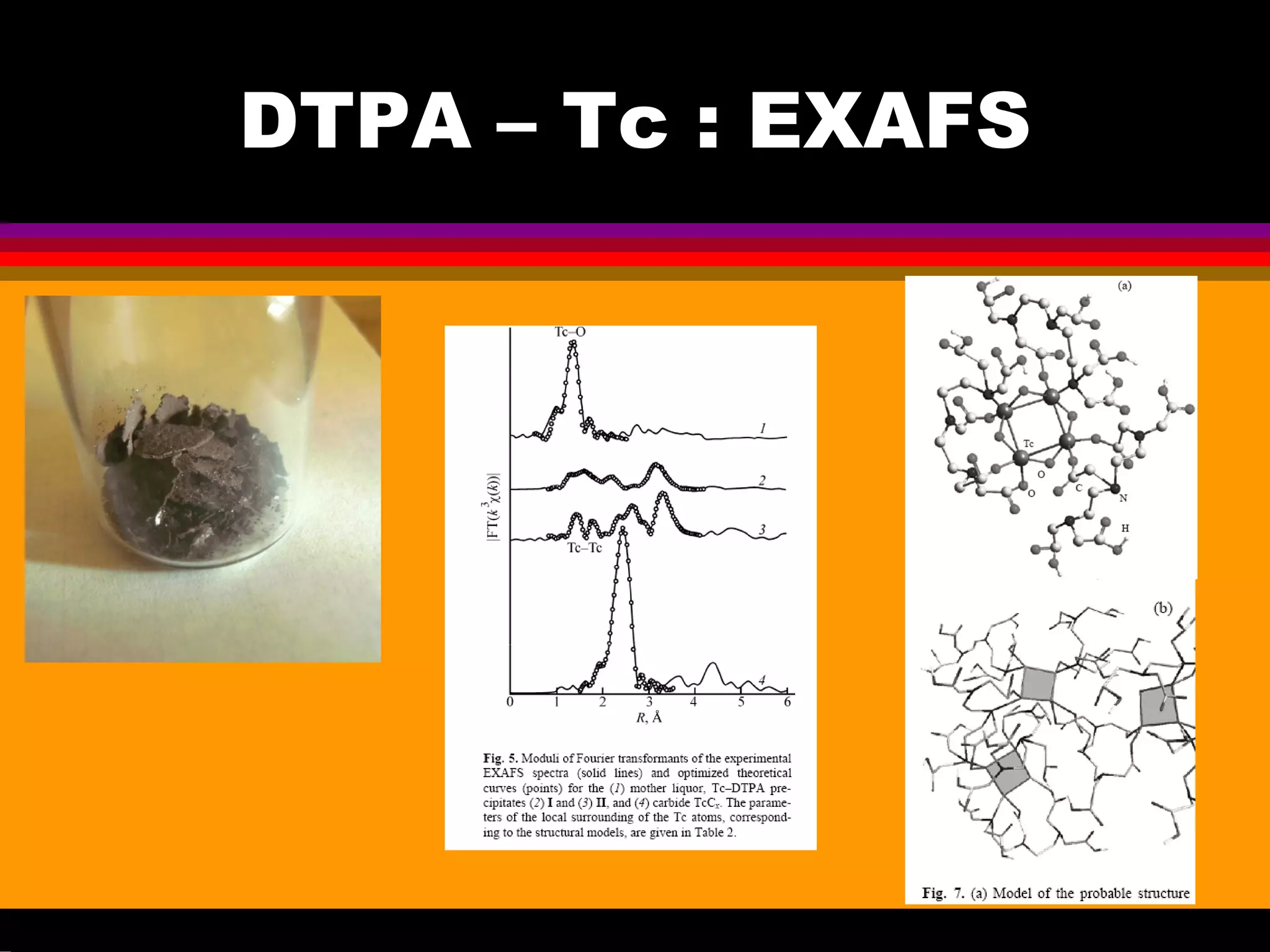
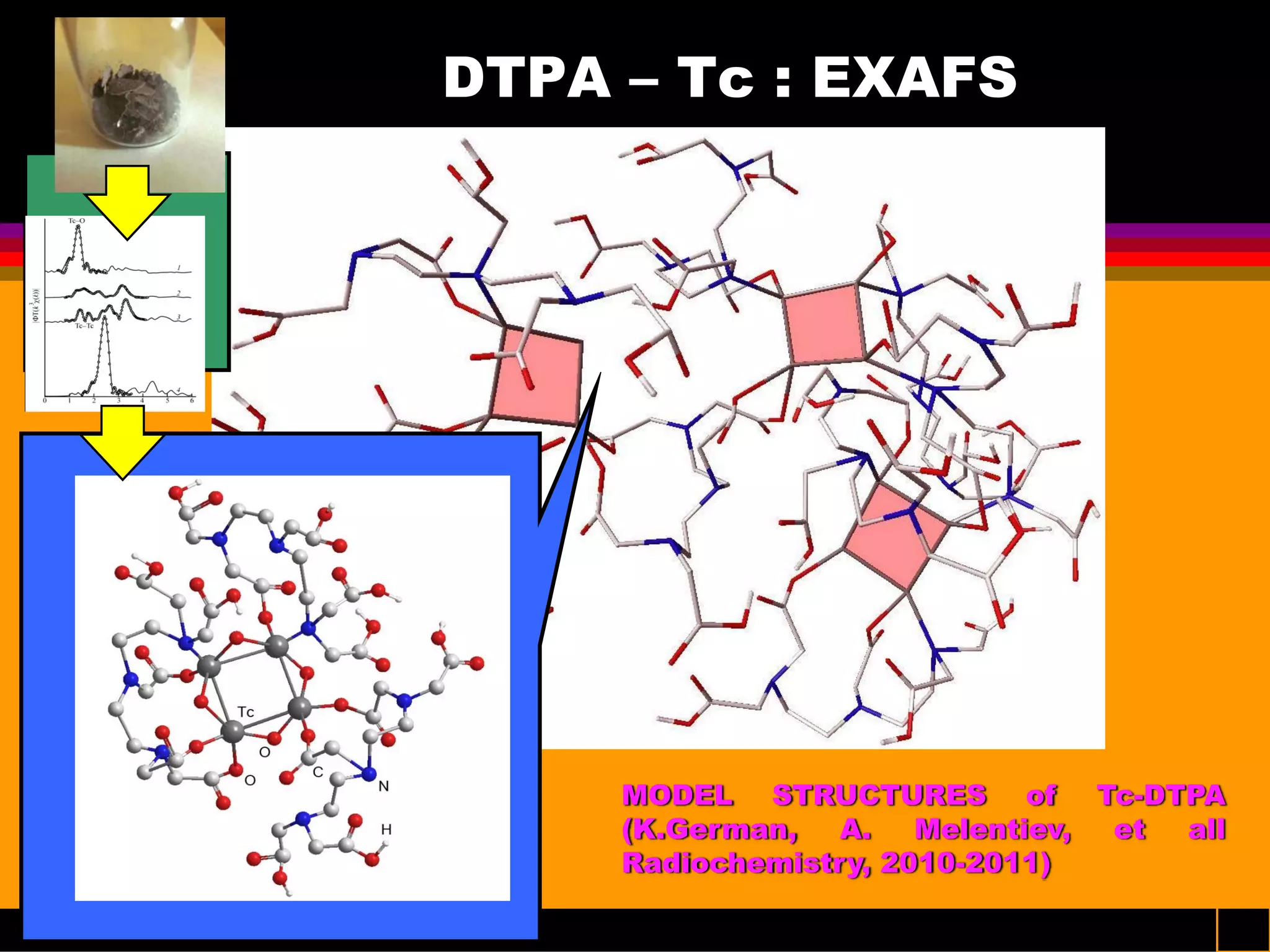
Interesting results were obtained with the Tc-DTPA complex formed under advanced PUREX conditions [6-7]. Great progress
have been achieved in the understanding of Tc(VII) behaviour in acids [8-10] that is important for explanation and prediction of Tc
and Re handling in acids, including the concentrated acid solutions up to highest. The investigation in crystal structures of Tc
compounds [2] enabled us with direct recommendations for the template synthesis for Tc and Re sensors [6]. The progress in Tc
carbonyl compounds gave chance for advanced Tc metal and Tc carbide films deposition [7]. Technetium sulphide and rhenium
were studied both with respect to medicine and to environmental behaviour of these elements [11]. The work on technetium
nanomaterials was carried in Russia in 2009-2010 within RFBR-09-03-00017, while the work on DTPA complexes with RFBR-09-
08000153.
References.
Peretrukhin V.F., German К.E., Маslennikov А.G. etc. Development of chemistry and technology of technetium. In.: «Modern
problems of physical chemistry» р. 681 – 695. М.: «Granitsy Publ.» (2005) 681-695.
Grigoriev M.S., German K.E., Maruk A.Y. // Acta Crystallogr. Sect E. (2007) V. 63. Pt.9. : P. m2061, and p. m2355.
Maruk A.Y. Grigoriev M.S., German K.E. Russ. Coord.Chem (2010) v.36, No 5, pp. 1–8.
Maruk A.Y. Grigoriev M.S., German K.E. Abstracts of the ”Conference on diffraction methods for substance investigations: from
molecules to crystals and nanomaterials”, Chernkgolovka. 30 june-3 july 2008. p.
Maruk A.Y. Grigoriev M.S., German K.E. Abstracts of the ”Conference on diffraction methods for substance investigations: from
molecules to crystals and nanomaterials”, Chernkgolovka. 25 june- 28 june 2010. p.
D.N. Tumanova, K.E. German, V.F. Peretrukhin, Ya.A. Obruchnikova, A.Yu. Tsivadze. Stabilization and spectral characteristics of
technetium and rhenium peroxides. In: 6-th International Symposium on Technetium and Rhenium. NMMU-Port Elizabeth, 7-10
October 2008, p.47.
D.N. Tumanova, K.E. German, V.F. Peretrukhin, A.Yu. Tsivadze. Formation of technetium peroxydes in anhydrous sulfuric acid.
Doklady Phys. Chem. 420 (2008) 114-117.
German K.E., Melentiev A.B., Kalmykov S.N., etc. Tc-DTPA sediments formed in technetium – hydrazine – DTPA – nitric acid
solutions. Journ. Nucl. Medcine and Biol.(2010). Sept. pp.
B.Ya. Zilberman. Radiochemistry , 42 (2000) 1-14.
Katayev E.A., Kolesnikov G.V., Khrustalev V.N. etc. // J. Radioanal. Nucl. Chem. (2009) 282: p. 385–389.
Maruk A.Y., German K.E., Kirakosyan G.A. etc. HtcO4. Abstracts of the 6-th Russian conference on radiochemistry, 12-16 Oct.
2009. Moscow. p.
F. Poineau, Ph. Weck, K. German, A. Maruk, G. Kirakosyan, W. Lukens, D. B. Rego, A. P. Sattelberger, K. R. Czerwinski . Speciation
of Heptavalent Technetium in Sulfuric Acid: Structural and Spectroscopic Studies. RSC-Dalton Transactions (2010) Dec. pp. (in
press).](https://image.slidesharecdn.com/18-09-2013technetiuminreprocessingofspentnuclearfuel-131005102207-phpapp02/75/Technetium-in-reprocessing-of-spent-nuclear-fuel-European-Summer-school-60-2048.jpg)