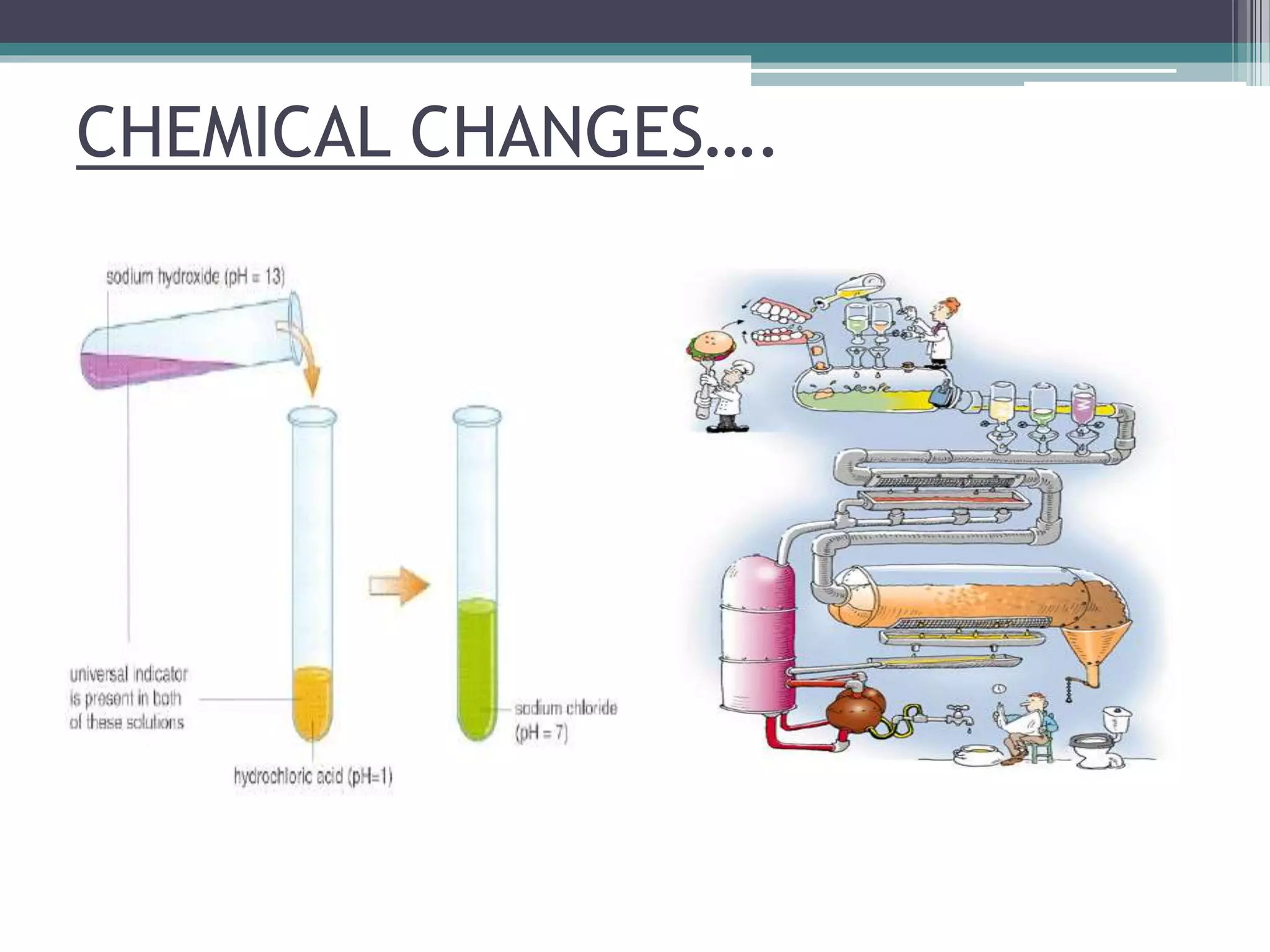
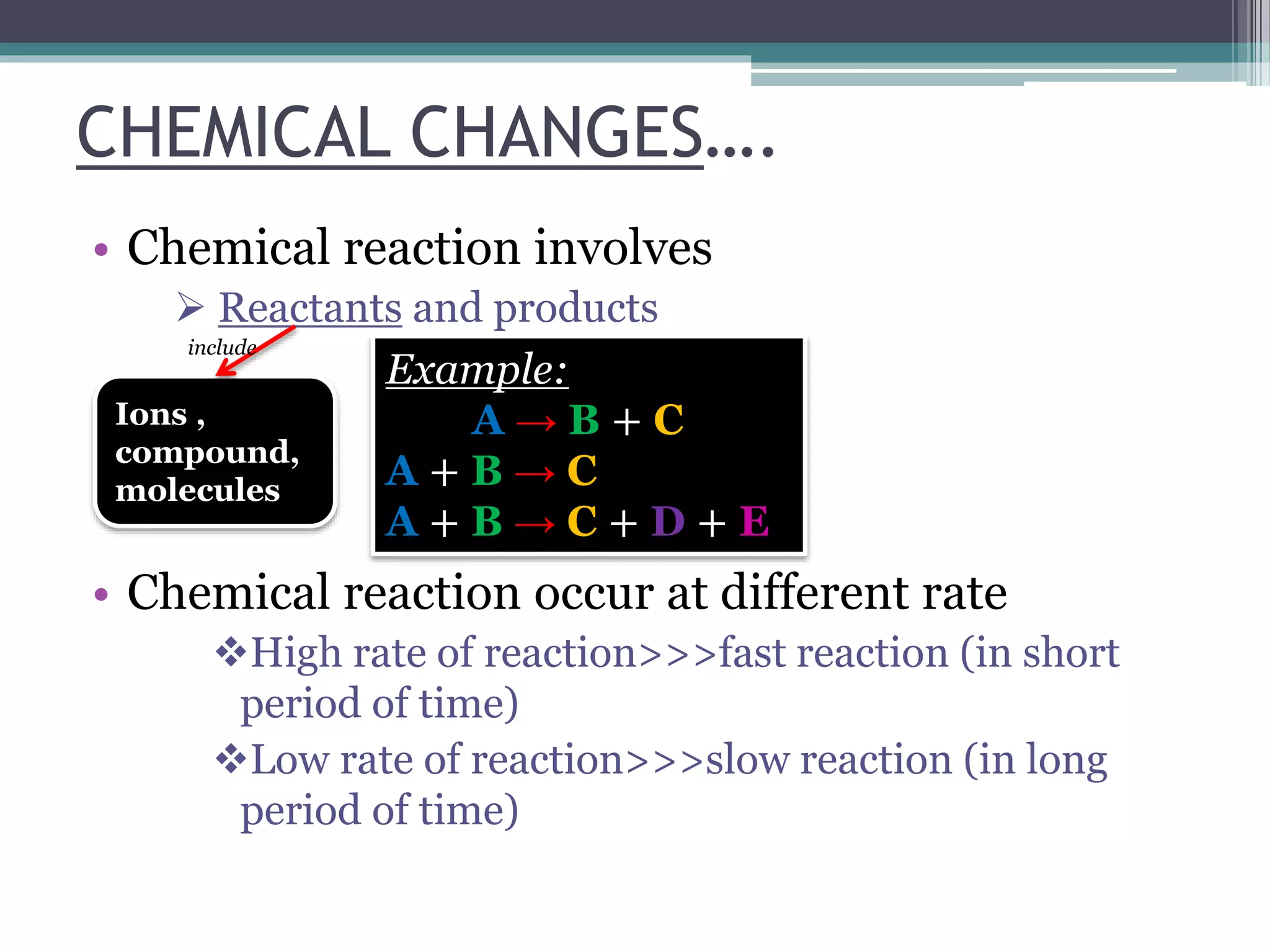
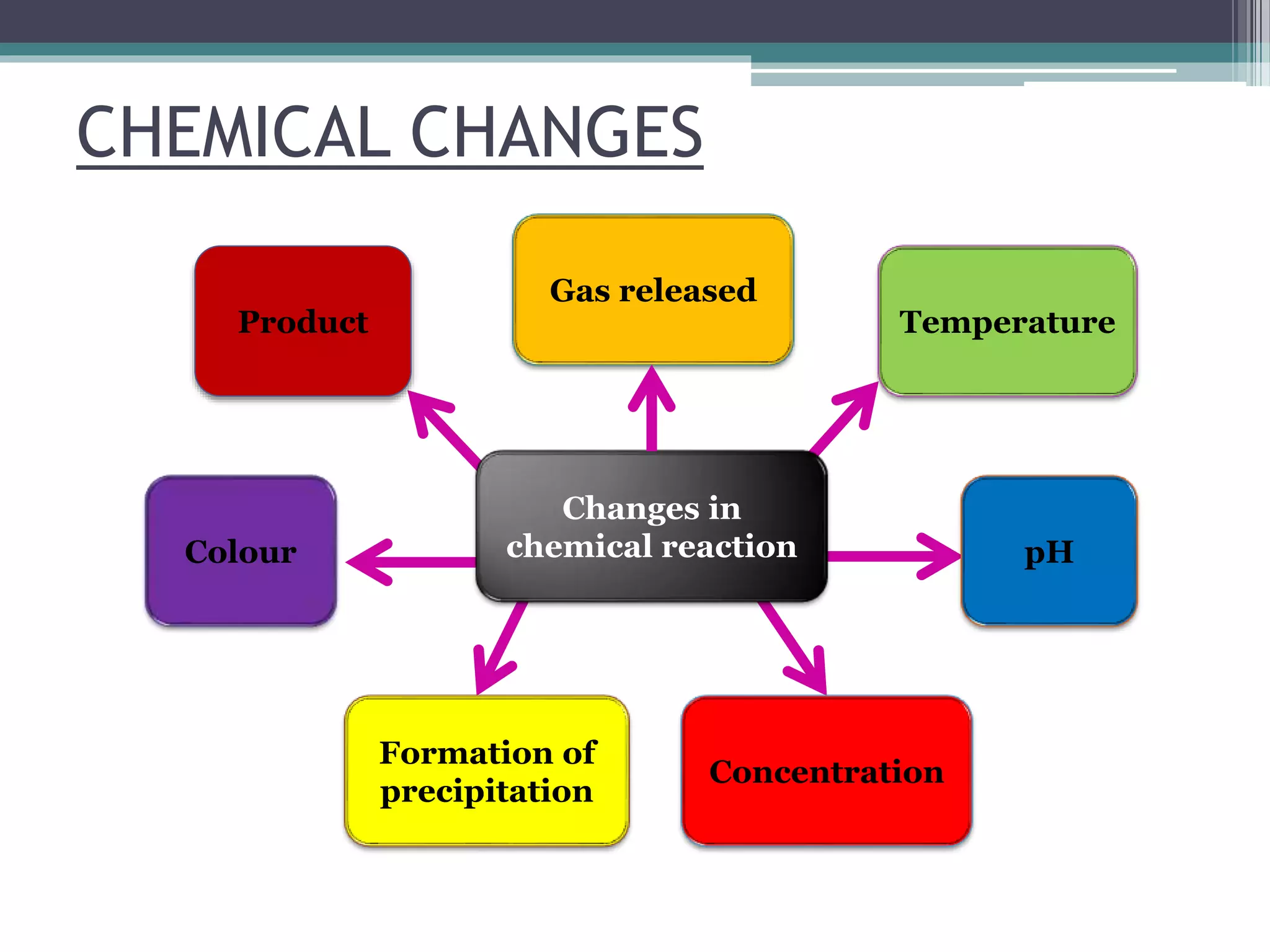
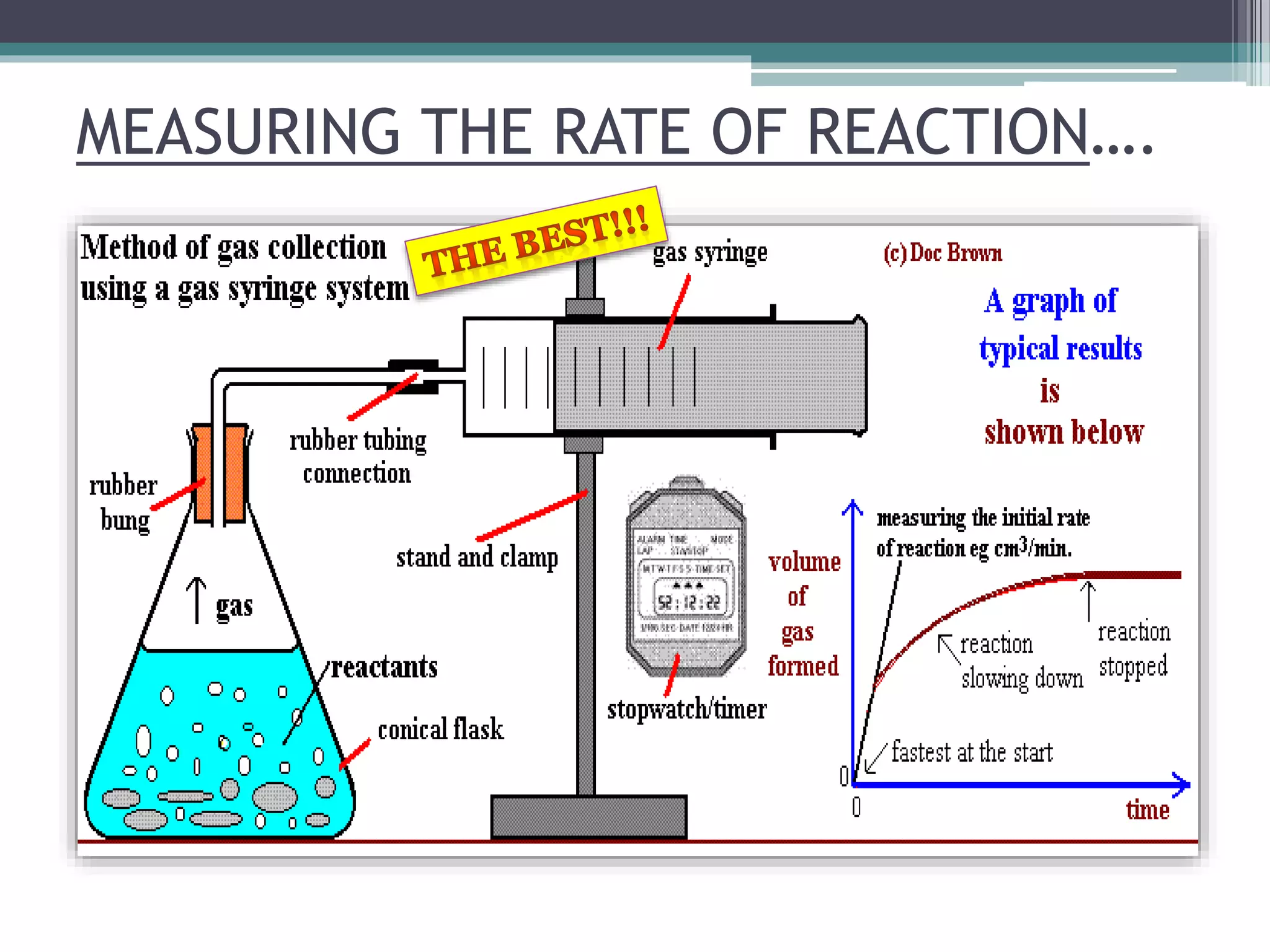
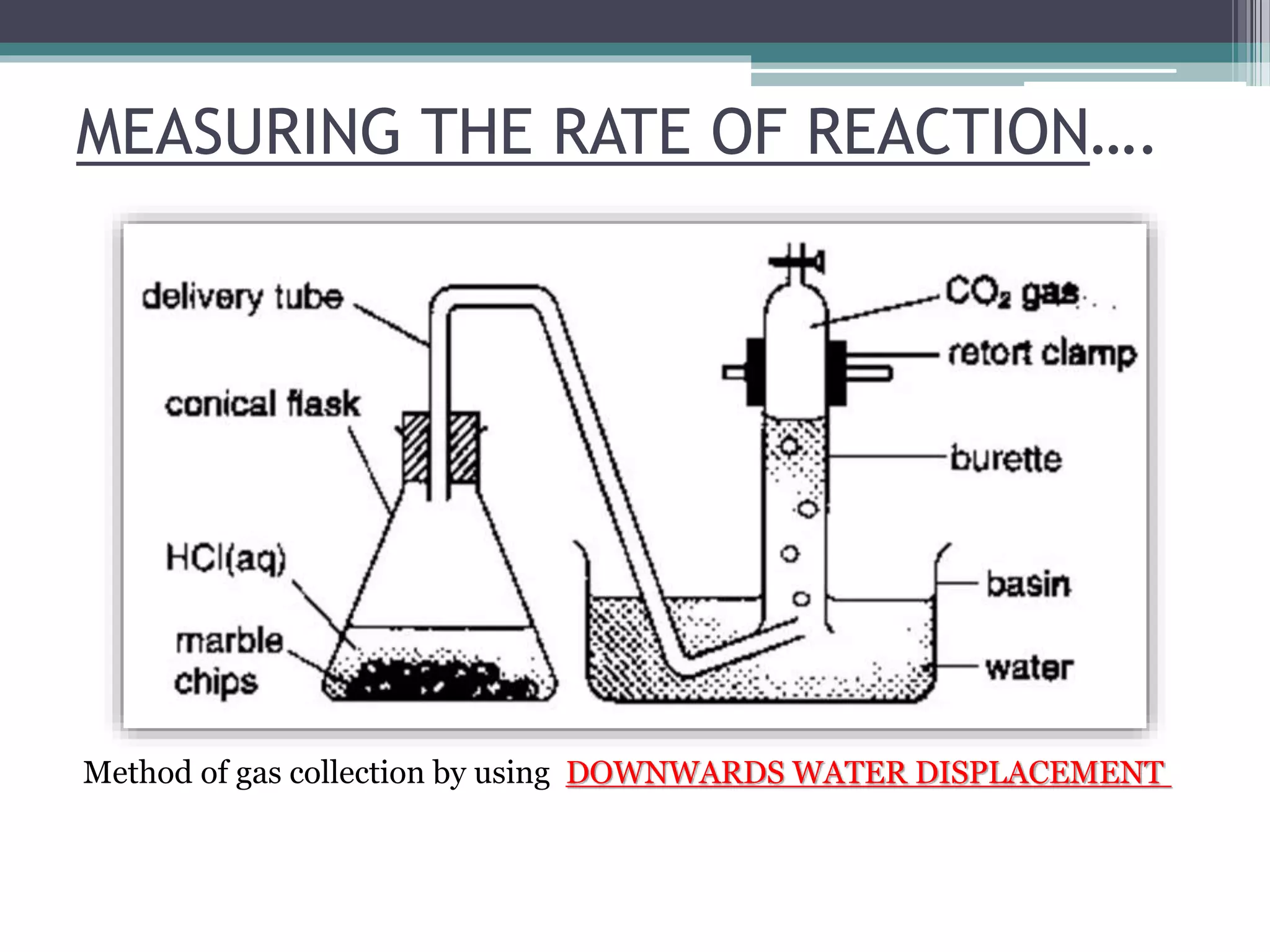
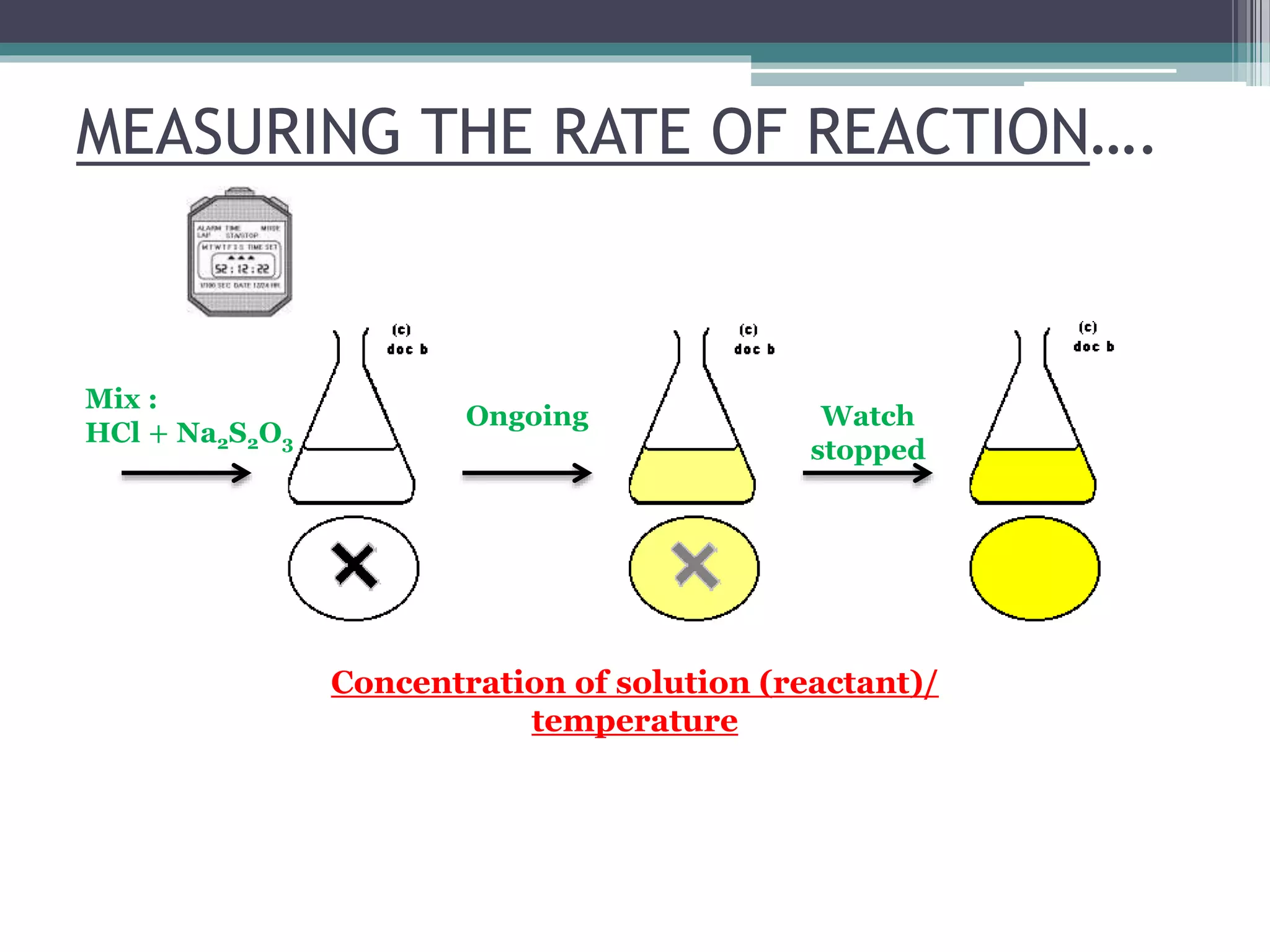
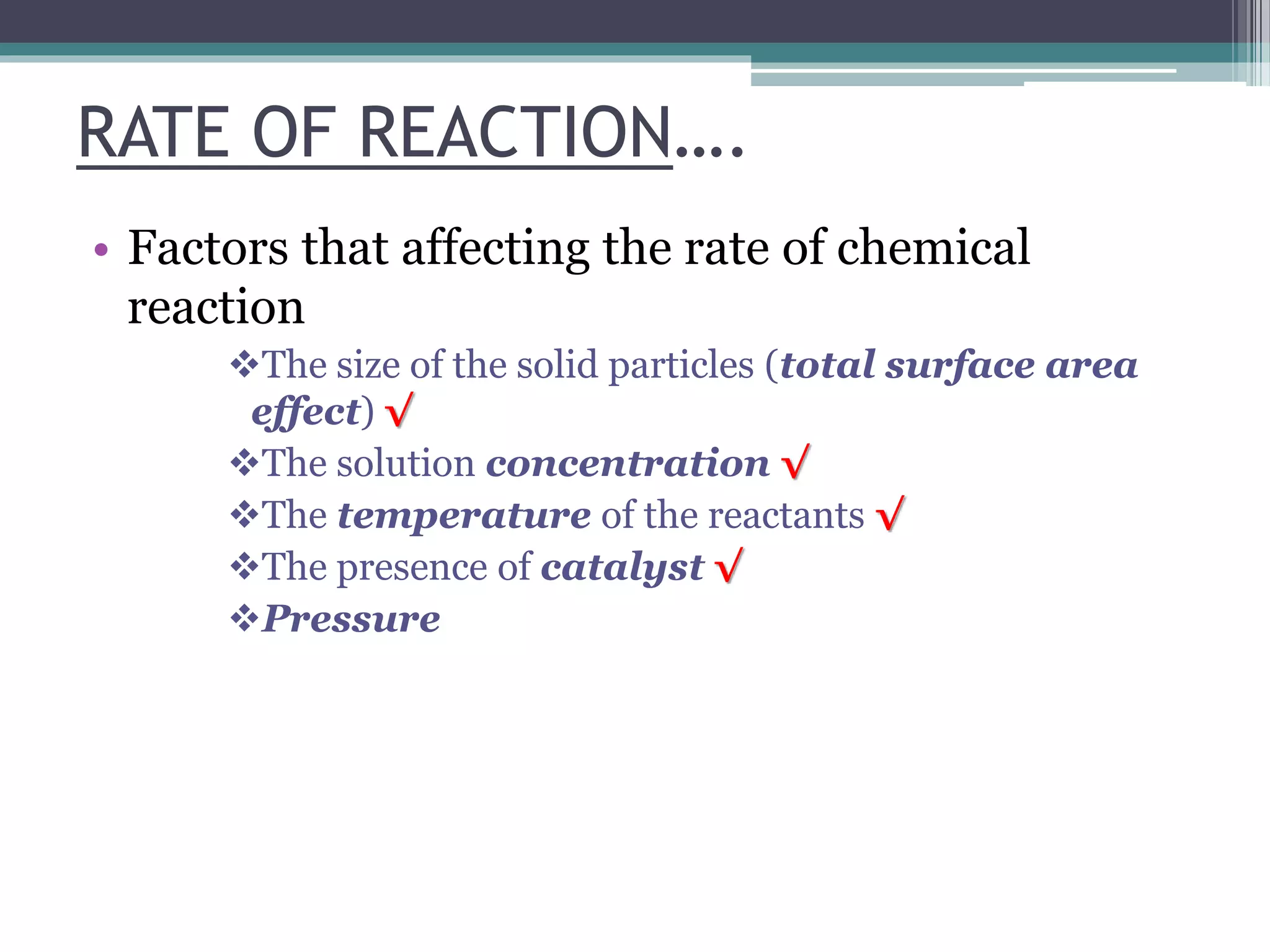
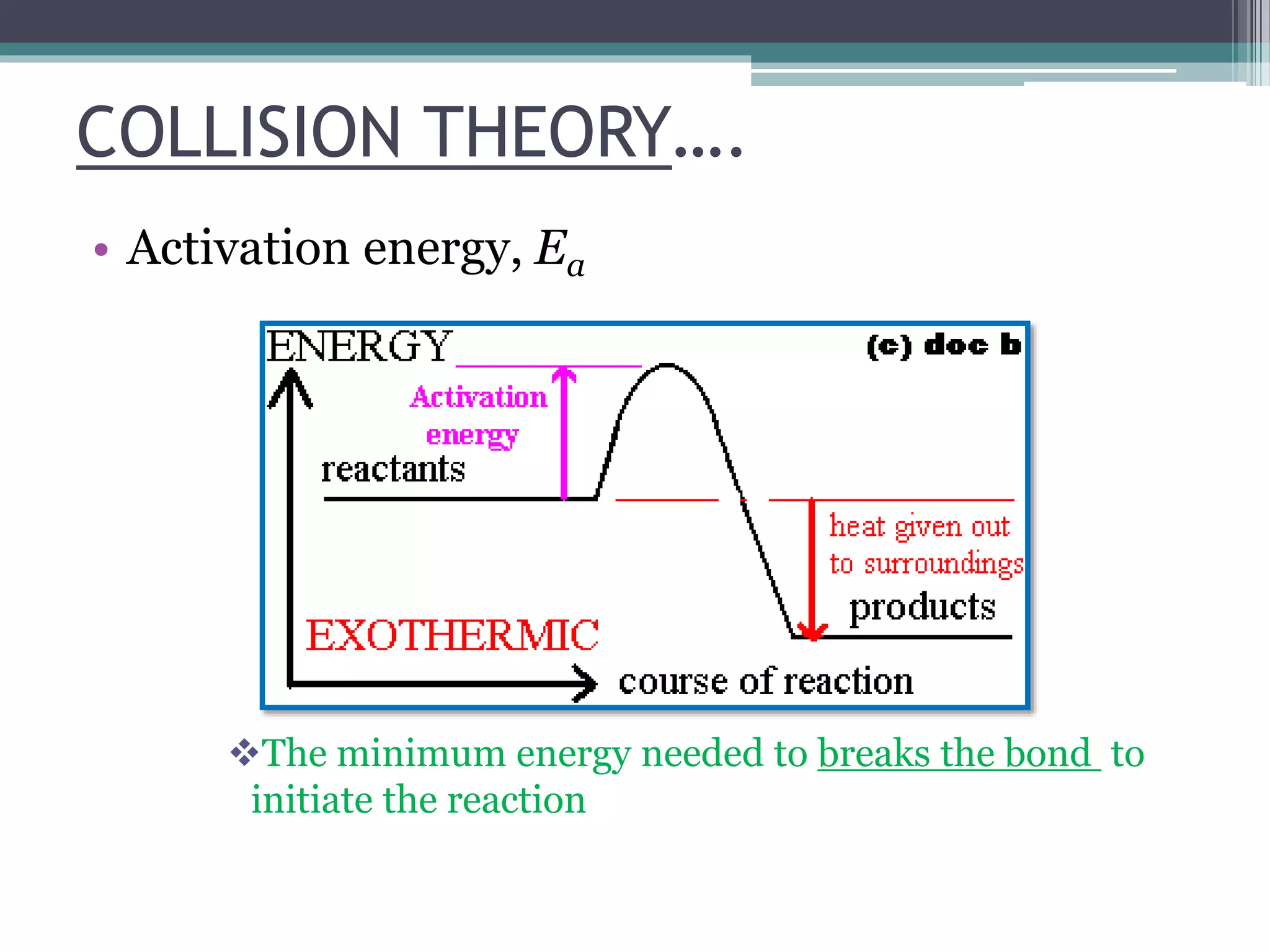
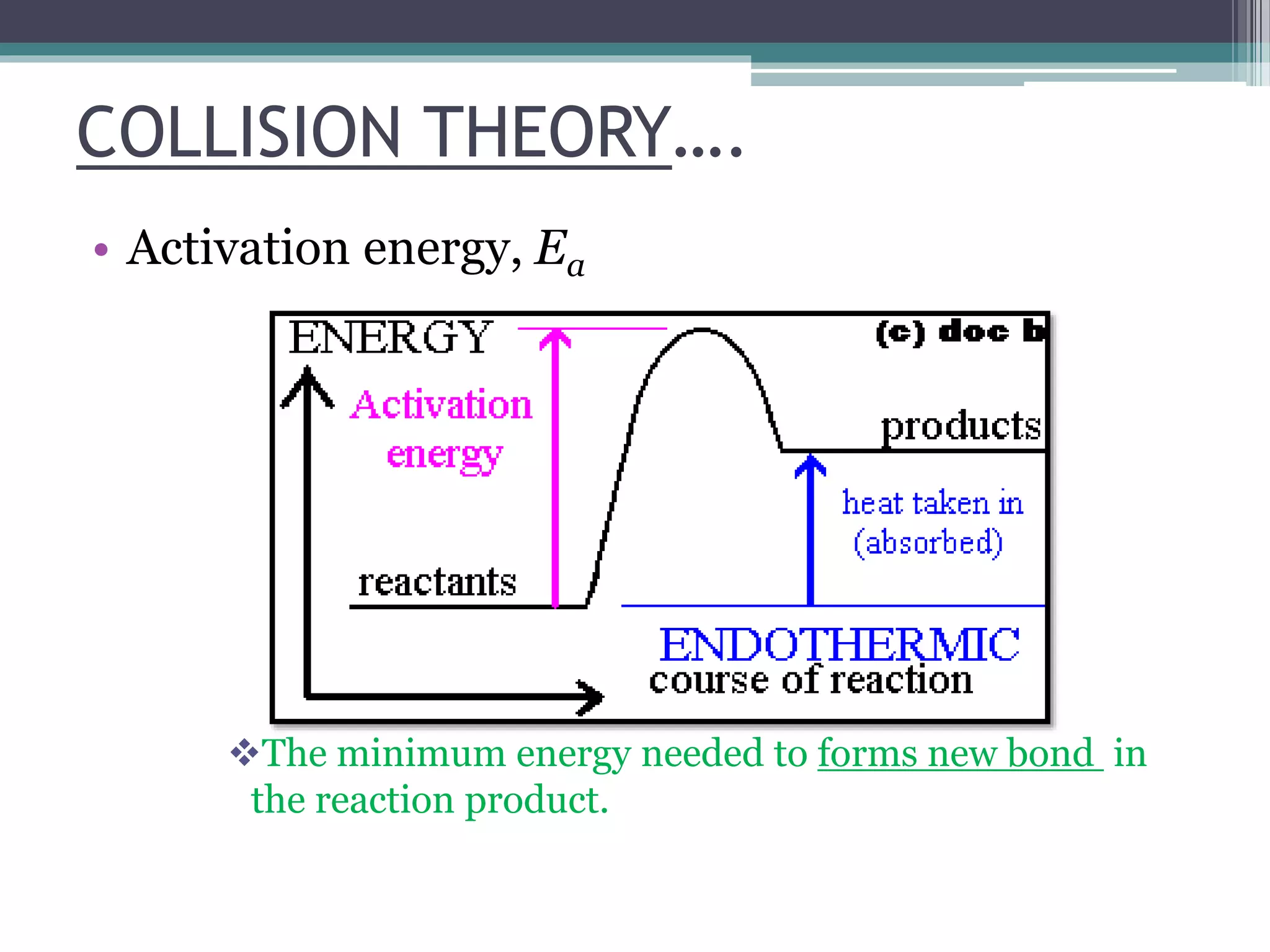
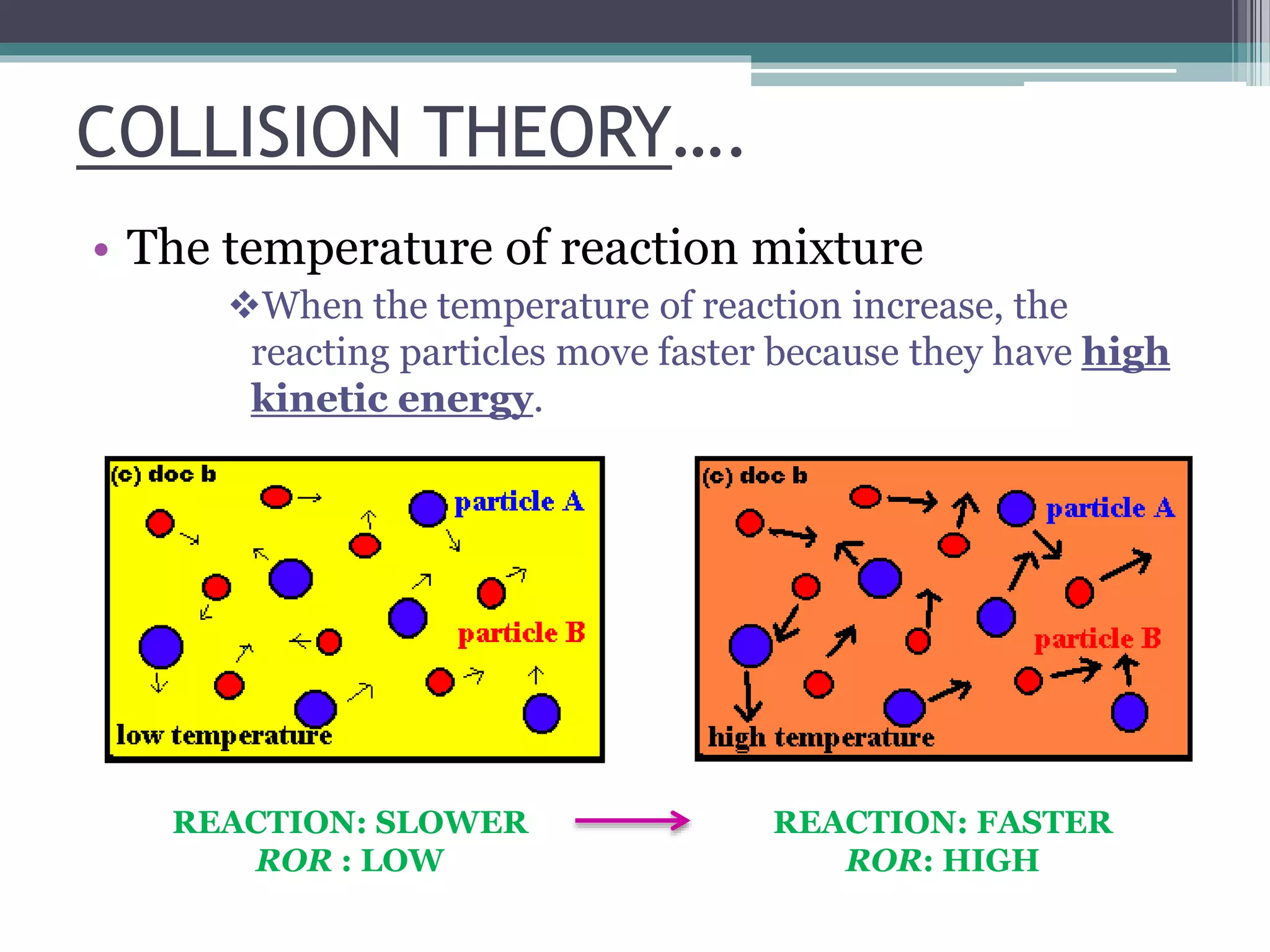
This document discusses factors that affect the rate of a chemical reaction. It defines the rate of reaction as the change in amount of reactant or product over time. The rate of reaction is affected by several factors, including the concentration of reactants, temperature, surface area, and presence of catalysts. A faster rate of reaction occurs when these factors increase the frequency of effective collisions between reactant particles that possess sufficient energy to overcome the activation energy barrier.