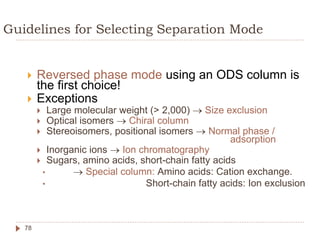
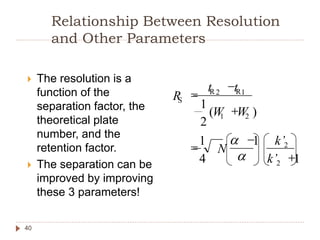
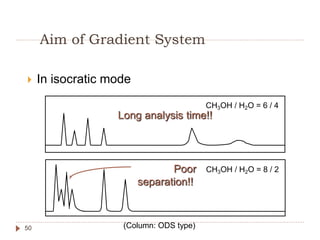
High Performance Liquid Chromatography (HPLC) is a technique used to separate, qualify, and quantify components in a sample through interactions between a mobile phase and a stationary phase. It offers advantages such as high sensitivity and the ability to analyze both polar and non-polar compounds without vaporization. The document discusses the principles, mechanisms, and equipment used in HPLC, along with comparisons to Gas Chromatography (GC) and methods for calibration and analysis.


















































![Four types of columns used in HPLC
1) High performance analytical columns
The internal diameter 1.0 - 4.6 mm; lengths 15 –250 mm] - used
mainly for qualitative and quantitative analysis
2) Preparative columns
The Internal Diameter > 4.6 mm; lengths 50 –250 mm) – used
mainly for preparative work
3) Capillary columns
The internal Diameter 0.1 -1.0 mm; various lengths)
4) Nano columns
the Internal diameter (< 0.1 mm)
HPLC Column](https://image.slidesharecdn.com/hadeiahplc-180330201556/85/hplc-53-320.jpg)