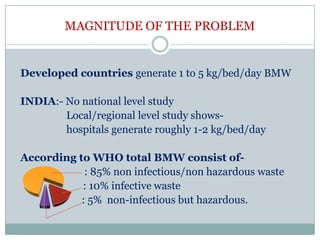
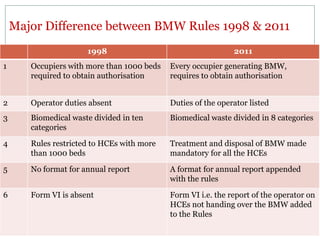
1. The document discusses India's Bio-medical Waste Management and Handling Rules. It outlines the need for proper management of biomedical waste to prevent disease transmission and protect public health.
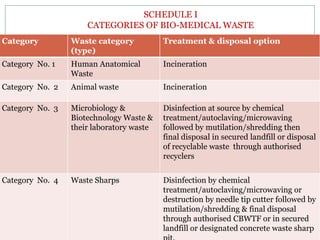
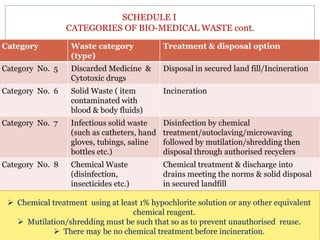
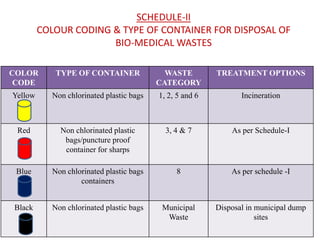
2. The rules categorize biomedical waste into 8 categories and specify treatment and disposal requirements for each category, such as incineration, autoclaving, or disposal in secured landfills.
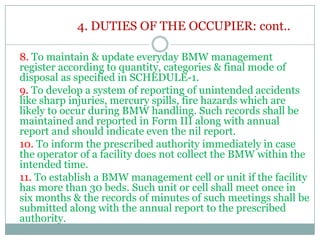
3. The duties of waste generators and operators are defined to ensure safe and responsible handling of biomedical waste from the point of generation through treatment and disposal. Proper segregation, packaging, transportation, and record-keeping are mandated.