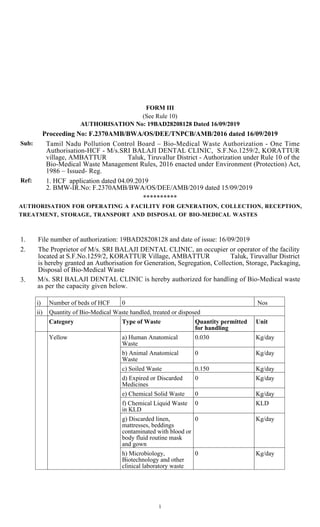
1. The document authorizes M/s. SRI BALAJI DENTAL CLINIC to operate a facility for handling bio-medical waste generated from their dental clinic located in Korattur village, Tiruvallur district, Tamil Nadu.
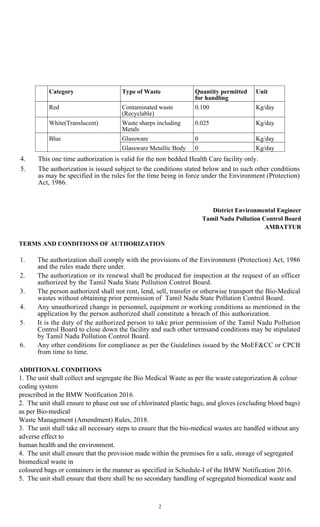
2. The authorization allows the clinic to generate, segregate, store, and dispose of up to 0.3 kg/day of human anatomical waste, 0.15 kg/day of soiled waste, and 0.025 kg/day of waste sharps.
3. The authorization is granted subject to compliance with various terms and conditions regarding proper segregation, storage, training, record-keeping, and ensuring the bio-medical waste