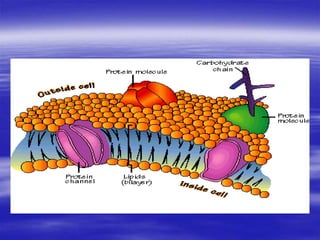
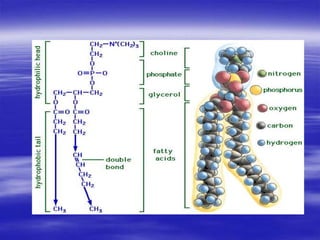
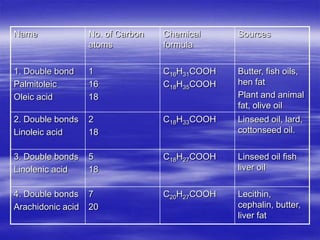
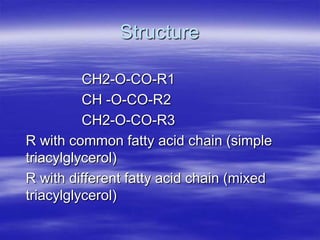
Lipids are organic compounds that include fats, oils, waxes, sterols and fat-soluble vitamins. They are made up of fatty acids and their derivatives and are soluble in organic solvents but not in water. Lipids include simple lipids like fats and oils which are esters of fatty acids and glycerol. They also include compound lipids like phospholipids and glycolipids which contain additional components like phosphate groups or carbohydrates. Lipids serve important functions like energy storage, insulation, cell membrane structure and transport of fat-soluble vitamins.