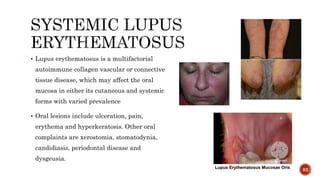
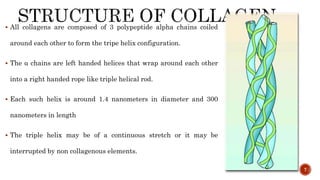
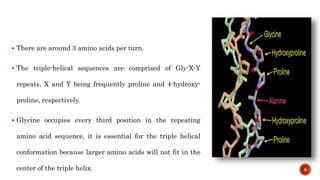
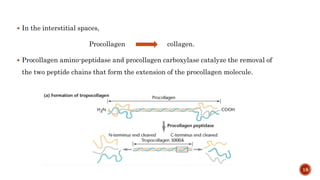
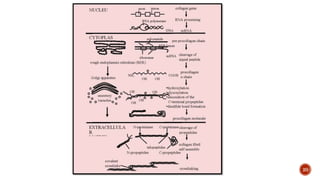
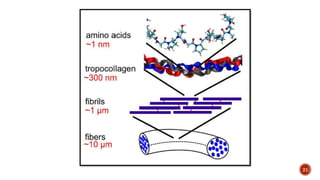
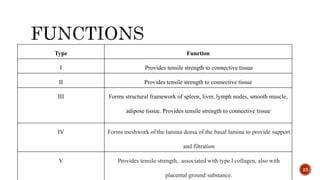
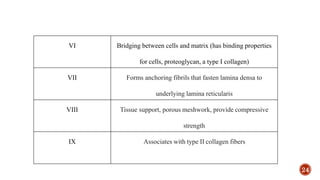
The document discusses the significance of collagen, the most abundant protein in mammals, detailing its structure, biosynthesis, and various types and functions. It highlights collagen's role in connective tissues, its biomedical applications, and the process of collagen degradation and remodeling, particularly in periodontal health. Additionally, it addresses the implications of collagen disorders and the use of collagen in tissue repair, drug delivery, and regenerative medicine.

























![40
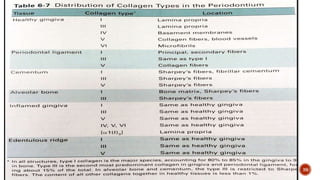
Out of 22 to 25% of organic component 94 to 98% is mainly collagen type I.
It contains type I collagen predominantly with the molecular configuration of
[α1 (I) α2 (I)].
During its formation in the osteoblast the large procollagen precursor undergoes
important post translational modifications. Suitably located proline and lysine
residues are hydroxylated to hydroxyproline and hydroxylysine respectively.](https://image.slidesharecdn.com/collagenandcollagendisorders-140621105723-phpapp01/85/Collagen-and-collagen-disorders-40-320.jpg)



























![Osteolathyrism is a collagen cross-linking deficiency caused by dietary
over-reliance on the seeds of Lathyrus sativus (kesari dal) in some
parts of India.
Osteolathyrogenic compounds like Beta-aminopropionitrile (BAPN) and
Beta-oxalyl aminoalanine [BOAA] found in Kesari dhal inhibit enzyme
lysyl oxidase required for the formation of cross links in the triple
helices
EFFECT
weakness and fragility of skin, bones, and blood vessels
Paralysis of the lower extremities associated with neurolathyrism
80](https://image.slidesharecdn.com/collagenandcollagendisorders-140621105723-phpapp01/85/Collagen-and-collagen-disorders-80-320.jpg)