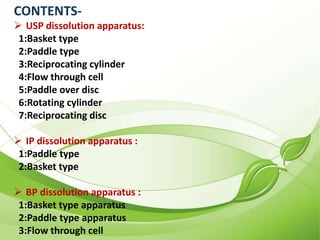
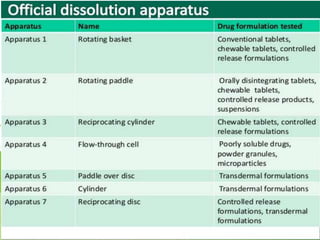
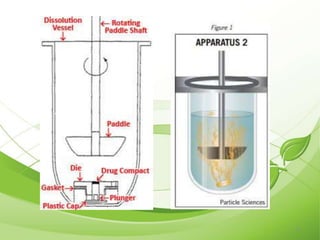
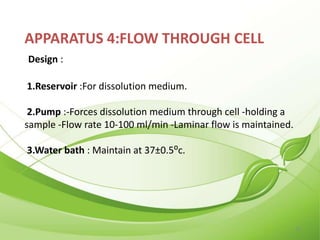
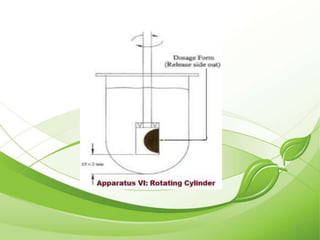
This document describes the various types of dissolution apparatus as specified by the USP, IP, and BP. It outlines 7 types of USP apparatus including the basket, paddle, reciprocating cylinder, flow through cell, paddle over disc, rotating cylinder, and reciprocating disc. The basket and paddle types are also included in the IP and BP. The key features and uses of each apparatus are provided along with diagrams. Ideal features of dissolution apparatus include precise specifications, simple design, sensitivity to changes, maintenance of sink conditions, and minimal dosage form abrasion.