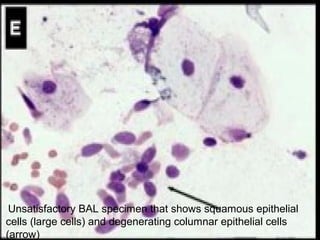
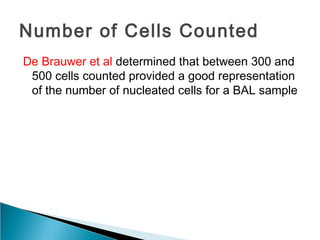
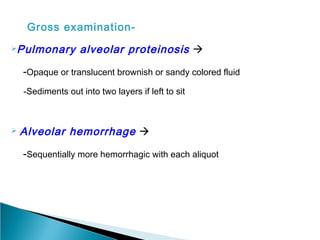
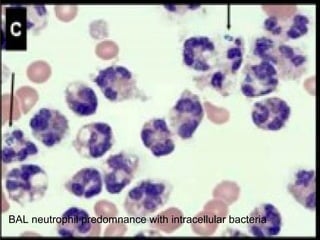
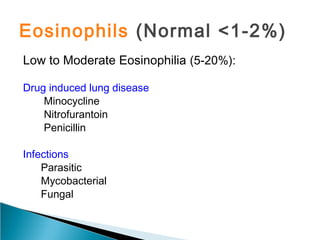
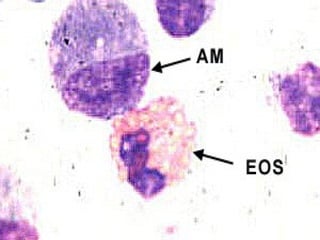
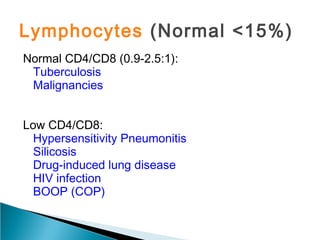
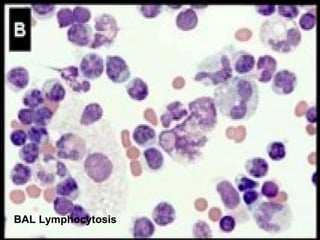
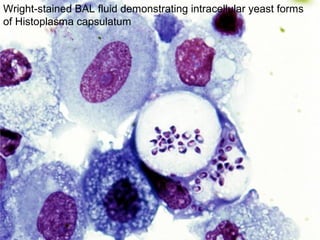
Broncho-Alveolar Lavage Fluid Analysis provides information on the status of the respiratory tract beyond what can be seen bronchoscopically. It involves instilling saline into segments of the lung and analyzing the cell types in the returned fluid. A satisfactory sample contains at least 2x10^6 cells including over 10 macrophages per field. Differential cell counts can indicate conditions like pneumonia, cancer, sarcoidosis, and alveolar hemorrhage. Special stains can identify pathogens, lipids, proteins, and minerals for diagnostic purposes. Complications are generally minor but loss of lung function is a risk in severely compromised patients.