DNA Topology Explained
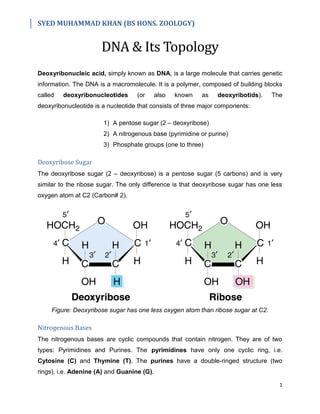
- 1. SYED MUHAMMAD KHAN (BS HONS. ZOOLOGY) 1 DNA & Its Topology Deoxyribonucleic acid, simply known as DNA, is a large molecule that carries genetic information. The DNA is a macromolecule. It is a polymer, composed of building blocks called deoxyribonucleotides (or also known as deoxyribotids). The deoxyribonucleotide is a nucleotide that consists of three major components: 1) A pentose sugar (2 – deoxyribose) 2) A nitrogenous base (pyrimidine or purine) 3) Phosphate groups (one to three) Deoxyribose Sugar The deoxyribose sugar (2 – deoxyribose) is a pentose sugar (5 carbons) and is very similar to the ribose sugar. The only difference is that deoxyribose sugar has one less oxygen atom at C2 (Carbon# 2). Figure: Deoxyribose sugar has one less oxygen atom than ribose sugar at C2. Nitrogenous Bases The nitrogenous bases are cyclic compounds that contain nitrogen. They are of two types: Pyrimidines and Purines. The pyrimidines have only one cyclic ring, i.e. Cytosine (C) and Thymine (T). The purines have a double-ringed structure (two rings), i.e. Adenine (A) and Guanine (G).
- 2. SYED MUHAMMAD KHAN (BS HONS. ZOOLOGY) 2 Figure: Cytosine and Thymine are single ringed pyrimidines that are present in the DNA molecules. Another single ringed pyrimidine is uracil, but it is not present in DNA, rather it is a part of the RNA molecules. Figure: Adenine and Guanine are double-ringed purines that are present in the DNA molecules. Phosphate Groups The phosphate groups are derived from phosphoric acid. Up to three phosphate groups can be present in a nucleotide. If one group is present, we call it a monophosphate, if two are present, we call it a diphosphate and if three are present, we call it a triphosphate. The nucleotides present in a DNA molecule have only one phosphate group attached to them (monophosphate) but the nucleotides present in the cytoplasm have three phosphates (triphosphate). Nucleotides & Nucleosides A nucleotide is formed when a pentose sugar (Deoxyribose, in case of a deoxyribonucleotide), nitrogenous base (Adenine, Guanine, Cytosine or Thymine in case of deoxyribonucleotide), and phosphate groups attached.
- 3. SYED MUHAMMAD KHAN (BS HONS. ZOOLOGY) 3 The part of a nucleotide that contains a nitrogenous base and a pentose sugar is called a nucleoside. In the case of DNA, it is a deoxyribonucleoside. The nucleosides have the suffix “ine” at the end of their names, i.e. Deoxyadenosine, Deoxyguanosine, Deoxycytidine, and Deoxythymidine. When phosphate groups are attached to these nucleosides, they become nucleotides, i.e. Deoxyadenosine monophosphate (dAMP), Deoxyguanosine monophosphate (dGMP), Deoxycytidine monophosphate (dCMP), and Deoxythymidine monophosphate (dTMP). The nucleotides present in the DNA molecules are in the form of monophosphates but those in the cytoplasm are in the form of triphosphates, i.e. Deoxyadenosine triphosphate (dATP), Deoxyguanosine triphosphate (dGTP), Deoxycytidine triphosphate (dCTP), and Deoxythymidine triphosphate (dTTP). This is because the DNA polymerase enzyme can only act upon triphosphates of deoxyribonucleotides. Figure: Structure of a nucleotide – Deoxyadenosine monophosphate (dAMP) The phosphate on the carbon 5 of deoxyribose of one nucleotide forms a bond with the hydroxyl group on carbon 3 of deoxyribose of another nucleotide. This bond is called a phosphodiester bond. Nucleotides in a chain of DNA are connected due to this bond.
- 4. SYED MUHAMMAD KHAN (BS HONS. ZOOLOGY) 4 Figure: Carbon 5 of one nucleotide and carbon 3 of another are linked together by a phosphodiester bond/linkage. The Equivalence Rule In 1950, Erwin Chargaff gave the equivalence rule. He said that the four nucleotide bases are not present in equal proportions in a DNA molecule. Instead, he proposed that the total number of purines was equal to the total number of pyrimidines: A+G = T+C The amount of adenine and thymine is equal (A=T) and the amount of guanine and cytosine is equal (G=C). This is because adenine forms a base pair with thymine, and guanine forms a base pair with cytosine. Physical Structure of DNA The physical structure of DNA was first understood when Rosalind Franklin obtained an X-ray diffraction photograph of DNA which demonstrated that DNA was a helical structure with a diameter of 20 Aº and a pitch (one round) of about 34 Aº. Based on Rosalind’s findings and Chargaff’s equivalence rule, Watson and Crick presented their model of DNA commonly known as the Watson and Crick model of DNA.
- 5. SYED MUHAMMAD KHAN (BS HONS. ZOOLOGY) 5 According to this model, in a DNA molecule, the adjacent deoxyribonucleotides are joined in a chain by phosphodiester bonds which link the 5' carbon of the deoxyribose of one mononucleotide unit with the 3' carbon of the deoxyribose of the next mononucleotide unit. According to Watson and Crick, DNA molecule consists of two polynucleotide chains wrapped helically around each other, with the sugar-phosphate chain on the outside (forming the ribbon-like backbone of double helix) and purines and pyrimidines on the inside of the helix. The two polynucleotide strands are held together by hydrogen bonds between specific pairs of purines and pyrimidines. Figure 1: Structure of DNA, according to Watson and Crick The hydrogen bonds between purines and pyrimidines are such that adenine can bond only with thymine by two hydrogen bonds, and guanine can bond only with cytosine by three hydrogen bonds. For every adenine in one chain, there will be thymine in the other and for every guanine in the first chain, there will be a cytosine in the other. Thus, the two chains are complementary to each other; that is, the sequence of nucleotides in one chain dictates the sequence of nucleotides in the other. The two strands run anti- parallel, i.e. in opposite directions. One strand has phosphodiester linkage in 3'→ 5' direction, while the other strand has phosphodiester linkage in just reverse or 5'→3' direction.
- 6. SYED MUHAMMAD KHAN (BS HONS. ZOOLOGY) 6 Both polynucleotides strands remain separated by 20 Aº distance. Ten mononucleotides occur per complete turn of DNA helix. The helix has two external grooves, a deep wide one, called major groove, and a shallow narrow one, called minor groove. Both of these grooves are large enough to allow protein molecules to come in contact with the bases. Forms of DNA There are three major forms of DNA, namely A, B, and Z DNA. They differ in the relation between their bases and the helical axis. B-DNA: It is right-handed DNA. It is the structure of fully hydrated DNA. It is the most common form of DNA encountered in vivo (inside a living system/cell). Its diameter is 20Ao and there are 10 nucleotides per one completer turn of 360o (meaning that the angle of twist of each nucleotide is 36o). The major groove is wide while the minor groove is narrow. A-DNA: It is also a right-handed DNA. This form of DNA is acquired when B-DNA is dehydrated, i.e. it is the dehydrated form of B-DNA. As a result of dehydration, the angle of twist of nucleotides increases. The diameter increases to 26Ao and the number of base pairs per 360o turn becomes 11.6. The major groove becomes narrow and deep while the minor groove becomes wide and shallow. The structural transformation of B- DNA to A-DNA (due to dehydration) is reversible. Z-DNA: It is a left-handed form of DNA. Z-DNA is so-called because of the zig-zag structure of its phosphate backbone. It has a diameter of 18Ao. There are 12 base pairs in a complete 360o turn. The minor groove is narrow and deep whereas the major groove is flat. Another important feature of Z-DNA is that adjacent nucleotides have opposite orientation, i.e. they form dinucleotides. Topology of DNA Topology is a branch of mathematics that deals with the study of the properties of geometric forms (shapes) that remain unchanged under certain transformations, as bending or stretching.
- 7. SYED MUHAMMAD KHAN (BS HONS. ZOOLOGY) 7 The ideal or relaxed state of DNA is the B form, a right-handed structure with a diameter of 20Ao. This is because this is the structure of minimum energy. Any deviation from this relaxed state causes an increase in energy, this problem is solved by the supercoiling of DNA, i.e. DNA curves itself into a coil. This minimizes the excess energy that builds up in the DNA molecule when it is deformed. 1. Linking Number The number of times that one strand wraps around the other remains fixed, it is known as the linking number. Linking number (denoted as Lk) is a measure of the total number of complete revolutions that either strand makes about the other. As long as the strands remain intact, Lk is a fixed quantity. The linking number cannot be changed unless a bond is broken. 2. Topoisomerases Topoisomerases are special enzymes that change the topology of DNA by changing its linking number by introducing a nick/cut in the DNA. There are two types of topoisomerases: Topoisomerase I and Topoisomerase II. Topoisomerase I changes Lk in units of one by breaking a single strand of DNA and allowing the duplex to unwind. Whereas, Topoisomerase II changes Lk by units of two by breaking both strands, creating a gate through which a second segment of helix is passed.
