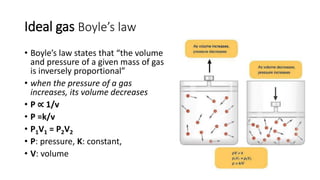
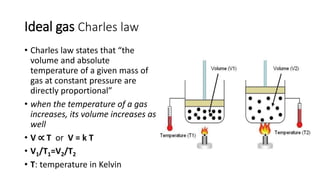
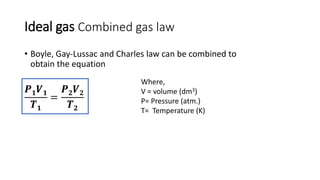
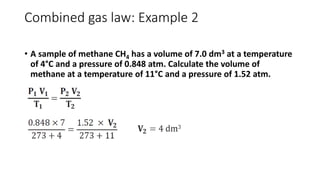
This document discusses the different states of matter and properties of gases. It begins by defining the four states of matter: gaseous, liquid, solid, and liquid crystalline. It then describes the general properties of gases, including their ability to expand infinitely and have low density. The document goes on to discuss ideal gases and the laws of Boyle, Charles, Gay-Lussac, and the combined gas law. It also covers the kinetic molecular theory and how the ideal gas law relates pressure, volume, temperature and moles of gas. Finally, it discusses how real gases differ from ideal gases and introduces the van der Waals equation to account for intermolecular forces.



![Gas general properties
• Gas molecules travel in random paths and collide with one another
and with the walls of the container in which they are confined
• Hence, gas exerts a pressure (a force per unit area) expressed in
dynes/cm2, atmospheres or in mmHg (1 atm = 760 mmHg = 760
Torr).
• Gases have volumes that is expressed in liters or cubic centimeters
(1 cm3 = 1 mL).
• The temperature involved in the gas equations is expressed by the
absolute or Kelvin scale [0°C = 273.15 K (Kelvin)].](https://image.slidesharecdn.com/gaseousstatesb-191018151625/85/Gaseous-State-SB-4-320.jpg)