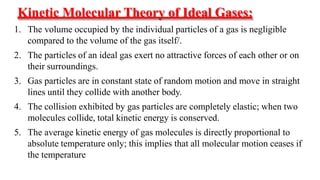
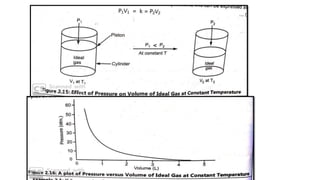
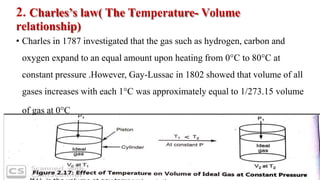
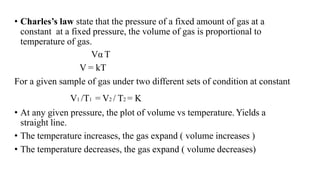
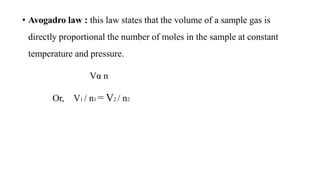
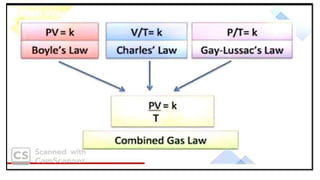
1) The document discusses the kinetic molecular theory of ideal gases and several gas laws including Boyle's law, Charles' law, Gay-Lussac's law, and Avogadro's law.
2) It explains that the ideal gas law relates the volume, pressure, temperature, and number of moles of a gas, and can be represented by the equation PV=nRT.
3) This law combines insights from earlier gas laws by showing that gas volume is directly proportional to the number of moles, temperature, and inversely proportional to pressure.