Embed presentation
Downloaded 294 times
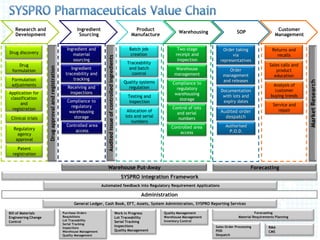

This document outlines the key processes involved in drug development and distribution including drug formulation, ingredient sourcing and traceability, quality management systems, warehouse management, order processing, regulatory compliance, and customer relationship management. It discusses stages from research and development through manufacturing, warehousing, sales, and quality control to ensure safety and meet regulatory requirements.
