Intro to chem
•Download as PPTX, PDF•
1 like•1,159 views
Matter is made up of atoms, which contain a nucleus of protons and neutrons surrounded by an electron cloud. Atoms are electrically neutral and bond with other atoms by sharing, losing, or gaining electrons in their outer valence shell. There are two main types of bonds: covalent bonds form when atoms share electrons, and ionic bonds form when one atom transfers an electron to another. Understanding the basic building blocks of matter and how atoms bond is fundamental to chemistry.
Report
Share
Report
Share
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
Similar to Intro to chem
Similar to Intro to chem (20)
chapter2-Atoms-Molecules-Ions.pp chemistry for pharmacyt

chapter2-Atoms-Molecules-Ions.pp chemistry for pharmacyt
More from Rachael Hubbard
More from Rachael Hubbard (20)
Recently uploaded
APM Welcome
Tuesday 30 April 2024
APM North West Network Conference, Synergies Across Sectors
Presented by:
Professor Adam Boddison OBE, Chief Executive Officer, APM
Conference overview:
https://www.apm.org.uk/community/apm-north-west-branch-conference/
Content description:
APM welcome from CEO
The main conference objective was to promote the Project Management profession with interaction between project practitioners, APM Corporate members, current project management students, academia and all who have an interest in projects.APM Welcome, APM North West Network Conference, Synergies Across Sectors

APM Welcome, APM North West Network Conference, Synergies Across SectorsAssociation for Project Management
This presentation was provided by William Mattingly of the Smithsonian Institution, during the fourth segment of the NISO training series "AI & Prompt Design." Session Four: Structured Data and Assistants, was held on April 25, 2024.Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"

Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"National Information Standards Organization (NISO)
This presentation was provided by William Mattingly of the Smithsonian Institution, during the third segment of the NISO training series "AI & Prompt Design." Session Three: Beginning Conversations, was held on April 18, 2024.Mattingly "AI & Prompt Design: The Basics of Prompt Design"

Mattingly "AI & Prompt Design: The Basics of Prompt Design"National Information Standards Organization (NISO)
Recently uploaded (20)
APM Welcome, APM North West Network Conference, Synergies Across Sectors

APM Welcome, APM North West Network Conference, Synergies Across Sectors
Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"

Mattingly "AI & Prompt Design: Structured Data, Assistants, & RAG"
Z Score,T Score, Percential Rank and Box Plot Graph

Z Score,T Score, Percential Rank and Box Plot Graph
Unit-IV; Professional Sales Representative (PSR).pptx

Unit-IV; Professional Sales Representative (PSR).pptx
Measures of Dispersion and Variability: Range, QD, AD and SD

Measures of Dispersion and Variability: Range, QD, AD and SD
Beyond the EU: DORA and NIS 2 Directive's Global Impact

Beyond the EU: DORA and NIS 2 Directive's Global Impact
Mattingly "AI & Prompt Design: The Basics of Prompt Design"

Mattingly "AI & Prompt Design: The Basics of Prompt Design"
SECOND SEMESTER TOPIC COVERAGE SY 2023-2024 Trends, Networks, and Critical Th...

SECOND SEMESTER TOPIC COVERAGE SY 2023-2024 Trends, Networks, and Critical Th...
Unit-V; Pricing (Pharma Marketing Management).pptx

Unit-V; Pricing (Pharma Marketing Management).pptx
ICT Role in 21st Century Education & its Challenges.pptx

ICT Role in 21st Century Education & its Challenges.pptx
Web & Social Media Analytics Previous Year Question Paper.pdf

Web & Social Media Analytics Previous Year Question Paper.pdf
Intro to chem
- 1. Introduction to Basic Chemistry
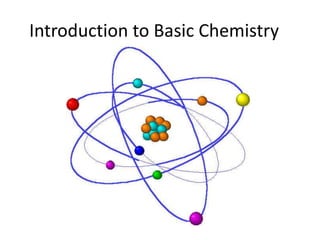
- 2. I. Building Blocks of Matter: MATTER is made up of the smallest BASIC particle called an ATOM (matter is anything that has mass…pretty much everything around you)
- 4. ENERGY LEVEL or ELECTRON CLOUD: area outside the nucleus that contains the electrons
- 6. II. Properties of Atoms Atoms are electrically NEUTRAL Number of ELECTRONS is equal to the number of PROTONS
- 7. 2. In nature, atoms will BOND to other atoms to become stable ELECTRONS are part of why atoms bond together
- 8. There are 2 types of electrons CORE and VALENCE electrons: ***Valence Electrons: OUTSIDE electrons that are involved in bonding.
- 9. 3. BONDING: an attraction within atoms to form a chemical compound All atoms are electrically neutral but are UNSTABLE until they form an attraction with another atom The outermost energy level may SHARE, LOSE, or GAIN electrons to form a BOND and become stable
- 10. Two types of bonds found within atoms: 1. Covalent: Atoms share electrons (can be single, double, or triple)
- 11. Two types of bonds found within atoms: 2. Ionic: When one or more electrons is lost from one atom and transferred to another
