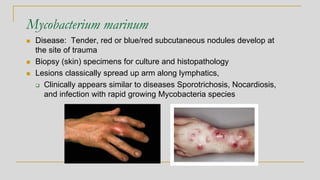
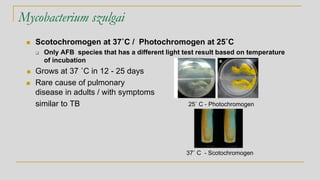
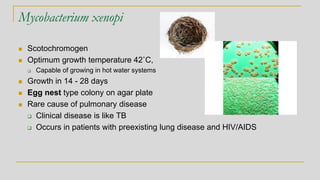
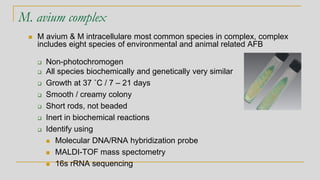
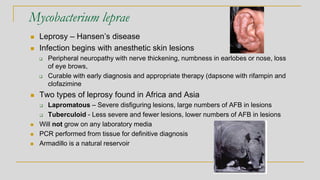
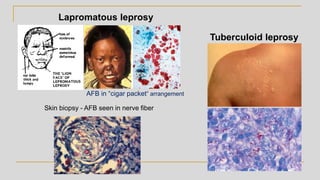
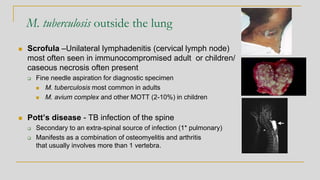
This document discusses mycobacteriology and the identification and testing of mycobacteria. It covers the following key points:
1. Mycobacteria are acid-fast bacilli that can be identified using genetic probes, MALDI-TOF mass spectrometry, or 16S rRNA sequencing. There are over 170 mycobacterial species separated into the TB complex and non-tuberculous mycobacteria.
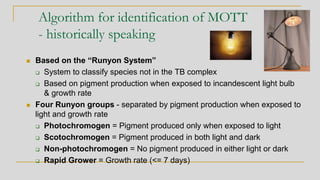
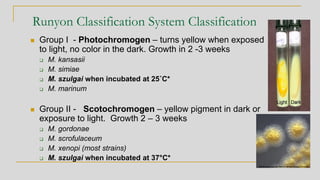
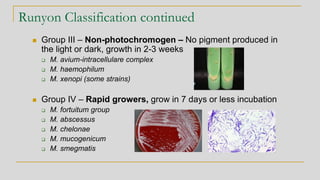
2. Identification of non-tuberculous mycobacteria was historically based on pigment production and growth rate, categorized using the Runyon system. Current best methods are genetic or proteomic.
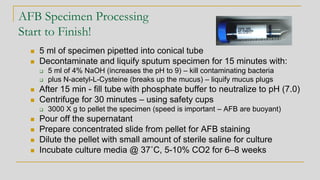
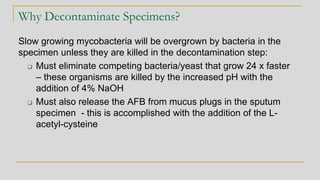
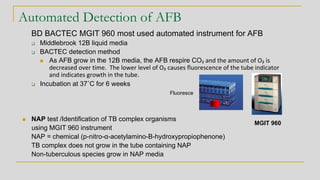
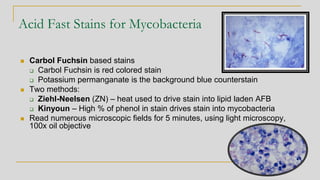
3. Specimen processing involves decontamination to eliminate competing bacteria, centrifug

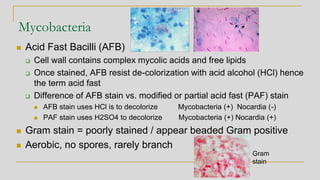






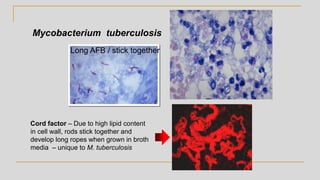
![Acid Fast Mycobacteria morphology
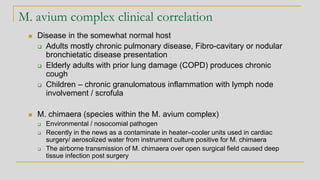
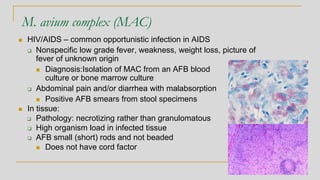
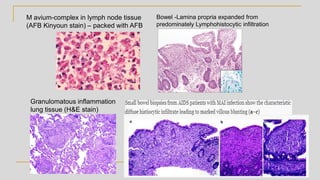
Mycobacterium avium complex
Organisms are routinely shorter than TB
(Kinyoun stain) without cording
M. tuberculosis - Organisms are long
rods and can appear as if they are sticking
together [cord factor]
In broth
cultures -
ropes of AFB
will form due to
cord factor](https://image.slidesharecdn.com/websitemycobacteriologyreview2019-190227173310/85/Mycobacteriology-Review-2019-21-320.jpg)
![Mycobacterium bovis
◼ Produces disease in cattle and other animals
❑ Spread to humans by raw milk ingestion
❑ Disease in humans similar to that caused by TB
◼ Can cause bladder infection in patients treated with BCG [Bacille Calmette-Guerin] /
used as an immune adjuvant to treat bladder cancer
❑ BCG is an attenuated strain of M. bovis, it can actibate and infect the bladder
❑ M. bovis isolated in culture from urine specimens
◼ Need to differentiate M. bovis from M. tuberculosis in such circumstances
M.bovis M. tb
Nitrate Negative Positive
Growth in T2H* No growth Growth
Pyrazinamidase enzyme Negative Positive
Pyrazinamide susceptibility Resistant Susceptible
*(Thiophene-2-carboxylic hydrazide)](https://image.slidesharecdn.com/websitemycobacteriologyreview2019-190227173310/85/Mycobacteriology-Review-2019-32-320.jpg)