308 Ethers.pdf
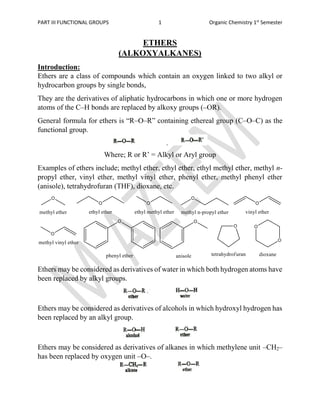
- 1. PART III FUNCTIONAL GROUPS 1 Organic Chemistry 1st Semester ETHERS (ALKOXYALKANES) Introduction: Ethers are a class of compounds which contain an oxygen linked to two alkyl or hydrocarbon groups by single bonds, They are the derivatives of aliphatic hydrocarbons in which one or more hydrogen atoms of the C–H bonds are replaced by alkoxy groups (–OR). General formula for ethers is “R–O–R” containing ethereal group (C–O–C) as the functional group. Where; R or R’ = Alkyl or Aryl group Examples of ethers include; methyl ether, ethyl ether, ethyl methyl ether, methyl n- propyl ether, vinyl ether, methyl vinyl ether, phenyl ether, methyl phenyl ether (anisole), tetrahydrofuran (THF), dioxane, etc. Ethers may be considered as derivatives of water in which both hydrogen atoms have been replaced by alkyl groups. Ethers may be considered as derivatives of alcohols in which hydroxyl hydrogen has been replaced by an alkyl group. Ethers may be considered as derivatives of alkanes in which methylene unit –CH2– has been replaced by oxygen unit –O–.
- 2. PART III FUNCTIONAL GROUPS 2 Organic Chemistry 1st Semester Structure: The oxygen atom of ethereal group (–O–) in ethers (alkoxyalkanes) is sp3 hybridized giving tetrahedral geometry to four sp3 hybridized orbitals around the oxygen atom. The oxygen atom forms two single covalent bonds with two carbon atoms; the C–O bonds (sp3-sp3 σ-bonds). The remaining two sp3 hybridized orbitals on oxygen atom are filled and nonbonding and act as two lone pairs of electrons. This results in a bent structure at oxygen atom. The C–O–C bond angle is approximately 110°. General structure of an ether is illustrated in figure. Classification: A. Based on Carbon Skeleton: 1. Aliphatic Ethers They have aliphatic carbon chain in their structure. They may be acyclic or cyclic. They may be saturated or unsaturated. They may be monoethers or polyethers. They may symmetrical or unsymmetrical. Ethers Aliphatic ethers Acyclic ethers Saturated Monoethers Symmetrical Unsymmetrical Polyethers Unsaturated Cyclic ethers Cyclic ethers Epoxides Crown ethers Aromatic ethers
- 3. PART III FUNCTIONAL GROUPS 3 Organic Chemistry 1st Semester Examples include methyl ether, ethyl ether, ethyl methyl ether, methyl n-propyl ether, vinyl ether, methyl vinyl ether, phenyl ether, tetrahydrofuran (THF), dioxane, dimethoxyethane (DME) etc. 2. Aromatic Ethers They have one or more aromatic rings in their structure and the ethereal oxygen is either directly attached with or part of the aromatic ring. They may symmetrical or unsymmetrical. Examples include phenyl ether, methyl phenyl ether (anisole), furan, etc. B. Based on Number of Ethereal Groups: 1. Monoethers: They have one ethereal group (C–O–C) in their molecules. They may be saturated or unsaturated. They may be aliphatic or aromatic. They may be acyclic or cyclic. They may be symmetrical or unsymmetrical. They are called Epoxides (oxiranes) if they are cyclic ethers with a three-atom ring containing the ethereal oxygen e.g., ethylene oxide, propylene oxide. Examples include methyl ether, ethyl ether, ethyl methyl ether, methyl n-propyl ether, vinyl ether, methyl vinyl ether, phenyl ether, tetrahydrofuran (THF), etc.
- 4. PART III FUNCTIONAL GROUPS 4 Organic Chemistry 1st Semester 2. Polyethers: They have two or more ethereal group (C–O–C) in their molecules. They may be acyclic or cyclic. They may be; o Crown Ethers; they are cyclic chemical compounds that consist of a ring containing several ether groups. o Other Polyethers; containing few to many ether groups. Examples include ethylene oxide, propylene oxide, dioxane, dimethoxyethane (DME), 18-crown-6, polyethylene glycol, etc. C. Based on Nature of Alkyl or Aryl Groups: 1. Symmetrical Ethers (Simple Ethers): They have two identical alkyl or aryl groups (–R) bonded to the oxygen atom. Examples include methyl ether, ethyl ether, vinyl ether, phenyl ether, etc. 2. Unsymmetrical Ethers (Mixed Ethers): They have two different alkyl or aryl groups (–R) bonded to the oxygen atom. Examples include ethyl methyl ether, methyl n-propyl ether, methyl vinyl ether, methyl phenyl ether (anisole), etc.
- 5. PART III FUNCTIONAL GROUPS 5 Organic Chemistry 1st Semester Nomenclature: The Common System: The unsymmetrical ethers are commonly named by giving the names of the two alkyl groups bonded to oxygen as separate words in alphabetical order and adding “ether” as the third word. The symmetrical ethers are named as “dialkyl ether”. But it is a common practice to omit the prefix “di”. In everyday laboratory language, the name “ether” when unspecified means diethyl ether. The IUPAC System: The IUPAC System names ethers as “alkoxyalkane”. In case of unsymmetrical ethers, one, the small alkyl group plus the oxygen atom (alkoxy group –OR) is treated as a substituent on the large alkyl group. Thus their systematic name is a one-word name. Isomerism in Ethers: The ethers exhibit Functional Isomerism, Chain Isomerism and Metamerism. Functional Isomerism: Ethers show functional isomerism with alcohols having the same carbon number. Thus each ether has one or more isomeric alcohols.
- 6. PART III FUNCTIONAL GROUPS 6 Organic Chemistry 1st Semester Chain Isomerism: Ethers show chain isomerism. Metamerism: Ethers show this type of isomerism due to the presence of unequal number of carbon atoms on either side of the ethereal oxygen. This enables simple ethers to be isomeric with mixed ethers. Methods of Preparation: 1. From Alcohols by Dehydration: a. With Concentrated Sulphuric Acid (conc. H2SO4): Symmetrical ethers are prepared by heating an alcohol with concentrated sulphuric acid. Two molecules of the alcohol eliminate a molecule of water to form ether. Note: The temperature is kept at 140°C and alcohol used in excess. If the temperature increases to 150°C, significant amounts of alkenes are obtained instead. b. With Alumina (Al2O3): In lower primary alcohols the dehydration may be effected by passing the vapours of the alcohol over alumina at 240-260°C. Note: The application of this method to secondary and tertiary alcohols is unsatisfactory owing to the marked tendency of these substances to form alkenes under the conditions.
- 7. PART III FUNCTIONAL GROUPS 7 Organic Chemistry 1st Semester c. With Diazomethane (CH2N2): Methyl ethers can be obtained by treatment of primary or secondary alcohols with diazomethane (CH2N2). Fluoroboric acid (HBF4) is used as a catalyst. 2. From Alkyl Halides: a. With Alcohols (Williamson’s Ether Synthesis): Ethers can be indirectly obtained from alcohols by converting them to alkoxides and then reacting them with alkyl halides. By taking a different alkyl halide, we can get mixed ether by this method. Note: This method is not applicable to tertiary alkyl halides because the alkoxide ions being both powerful nucleophiles and bases would bring dehydrohalogenation of the tertiary alkyl halides to form alkenes preferentially. b. With Silver Oxide (Ag2O): Simple ethers can be obtained by boiling alkyl halides with dry silver oxide (Ag2O). By taking a mixture of two different alkyl halides the method can be used for the formation of mixed ethers. 3. From Halogenated Ethers with Grignard’s Reagents: This is a good method for preparing higher ethers from lower members.
- 8. PART III FUNCTIONAL GROUPS 8 Organic Chemistry 1st Semester Physical Properties: Physical State/Odour/Taste: Excepting dimethyl ether and ethyl methyl ether both of which are gases, all ethers are colourless liquids with pleasant odours. Anesthetic Action: Lower ethers act as anesthetics. Lower ethers are highly volatile and very flammable. Solubility: Ethers are slightly soluble in water. This is attributed to formation of hydrogen bonds between water and the ethereal oxygen. Their specific gravities show a gradual increase with increase in molecular weights but they are lighter than water. The boiling points increase with increasing molecular weight. Ethers have lower boiling points than isomeric alcohols due to lack of hydrogen bonding in ethers. The boiling points of ethers are slightly higher but close to the boiling points of alkanes with same number of atoms. Chemical Properties (Reactions): Inertness of Ethers: Owing to the absence of active groups and multiple bonds from their molecules, ethers are comparatively inert substances. The reagents like ammonia, alkalis, dilute acids and metallic sodium have no action upon them in cold. They are not readily oxidized or reduced. Reactivity of Ethers: Reactivity of ethers can be attributed to polar C–O bonds as well as to availability of lone pairs of electrons on the ethereal oxygen atom. Chemical reactions of ethers can be categorized as; Reactions involving alkyl groups (substitution reactions). Reactions involving the unshared electrons (lone pairs) of oxygen atom. Reactions involving rupture of the C–O bond (decomposition reactions). A. Reactions of Ethers due to Alkyl Groups (Substitution Reactions): 1. Halogenation: Ethers when heated with chlorine or bromine undergo halogenation, the extent of which depends upon the reaction conditions. Halogenation preferentially takes place at alpha carbon atoms.
- 9. PART III FUNCTIONAL GROUPS 9 Organic Chemistry 1st Semester In the presence of light perchlorodiethyl ether is obtained. B. Reactions of Ethers due to Ethereal Oxygen: 1. Formation of Peroxides (Autoxidation): In contact with air or ozone, ethers form peroxides. The formation of peroxides is accelerated by ultraviolet light and by the absence of moisture. 2. Formation of Oxonium Salts (Basic Character): Ethers like alcohols are weakly basic and react with strong acids (e.g., H2SO4, HClO4, HBr) to form oxonium salts which are unstable when diluted with water. Ethers form relatively stable complexes with Lewis acids (e.g., BF3 and RMgX) by coordination.
- 10. PART III FUNCTIONAL GROUPS 10 Organic Chemistry 1st Semester C. Reactions of Ethers due to C–O Bond (Decomposition Reactions): 1. Formation of Alcohols by Hydrolysis: a. By Action of Water (H2O): When boiled with water or treated with steam, ethers are decomposed at one of the oxygen bonds to form alcohols. Hydrolysis is accelerated by acids. b. By Action of Sulphuric Acid (H2SO4): Cold concentrated sulphuric acid has no action on ethers except that it dissolves them forming oxonium salts. However, if the solution is heated, the ether is decomposed to yield a molecule of the alcohol and alkyl hydrogen sulphate. 2. Formation of Alkyl Halides: a. By Action of Halogen Acids (HX): Ethers are readily cleaved by hydroiodic acid (HI) or hydrobromic acid (HBr). The final products of reaction are governed by the reaction conditions. In cold, ethers split to form a molecule of alkyl halide and alcohol. In mixed ethers, the halogen goes with the smaller alkyl group. In hot and with excess of halogen acid, both alkyl groups appear as alkyl halides. b. By Action of Phosphorous Pentachloride (PCl5): Ethers react with phosphorous pentachloride (PCl5) to give alkyl chlorides.
- 11. PART III FUNCTIONAL GROUPS 11 Organic Chemistry 1st Semester c. By Action of Acetyl Chloride: Ethers react with acetyl chloride in the presence of anhydrous zinc chloride (ZnCl2) to form alkyl chloride and alkyl acetate. APPLICATIONS AND USES (SIGNIFICANCE): A. INDUSTRIAL/COMMON USES 1. Ethers are used as laboratory solvents as well as solvents for fats, waxes, oils, varnishes, dyes etc. 2. Vapours of certain ethers are used as insecticides, miticides, and fumigants for soil. 3. Dimethyl ether is used as a spray propellant and refrigerant. 4. Methyl t-butyl ether (MTBE) is a gasoline additive that boosts the octane number and reduces the amount of nitrogen-oxide pollutants in the exhaust. 5. The ethers of ethylene glycol are used as solvents and plasticizers. 6. Ethers are also used in polymerization reactions such as paraformaldehyde, polyethylene glycol (PEG), polypropylene glycol (PPG), polyphenyl ether (PPE) etc. 7. Ethylene oxide is used as a fumigant and to make antifreeze, ethylene glycol, and other useful compounds. 8. Epoxides can be used to assemble polymers known as epoxies, which are excellent adhesives and useful surface coatings. 9. Many inorganic salts can be made soluble in nonpolar organic solvents by complexing them with an appropriate crown ether. B. PHARMACEUTICAL/CLINICAL USES 10.Ethers are used as antiseptic and disinfectants. 11.Diethyl ether is used as general anesthetic 12.Codeine, a potent pain-relieving drug, is the methyl ether of morphine. 13.Butylated hydroxyanisole (BHA) is used as an antioxidant. C. NATURAL OCCURANCE 14.Ethers are they are common linkages in carbohydrates and lignins. 15.Anisole is an aryl ether and a major constituent of the essential oil of anise seed used in perfumes & cosmetics. 16.Anisole is also a component of many insect pheromones. 17.Anisole and anethol are ethers found in fennel.
- 12. PART III FUNCTIONAL GROUPS 12 Organic Chemistry 1st Semester 18.Some toxins produced by dinoflagellates such as brevetoxin and ciguatoxin are extremely large and are known as cyclic or ladder polyethers. Anisole Anethol Butylated hydroxyanisole (BHA) Paraformaldehyde Brevetoxin A Ciguatoxin