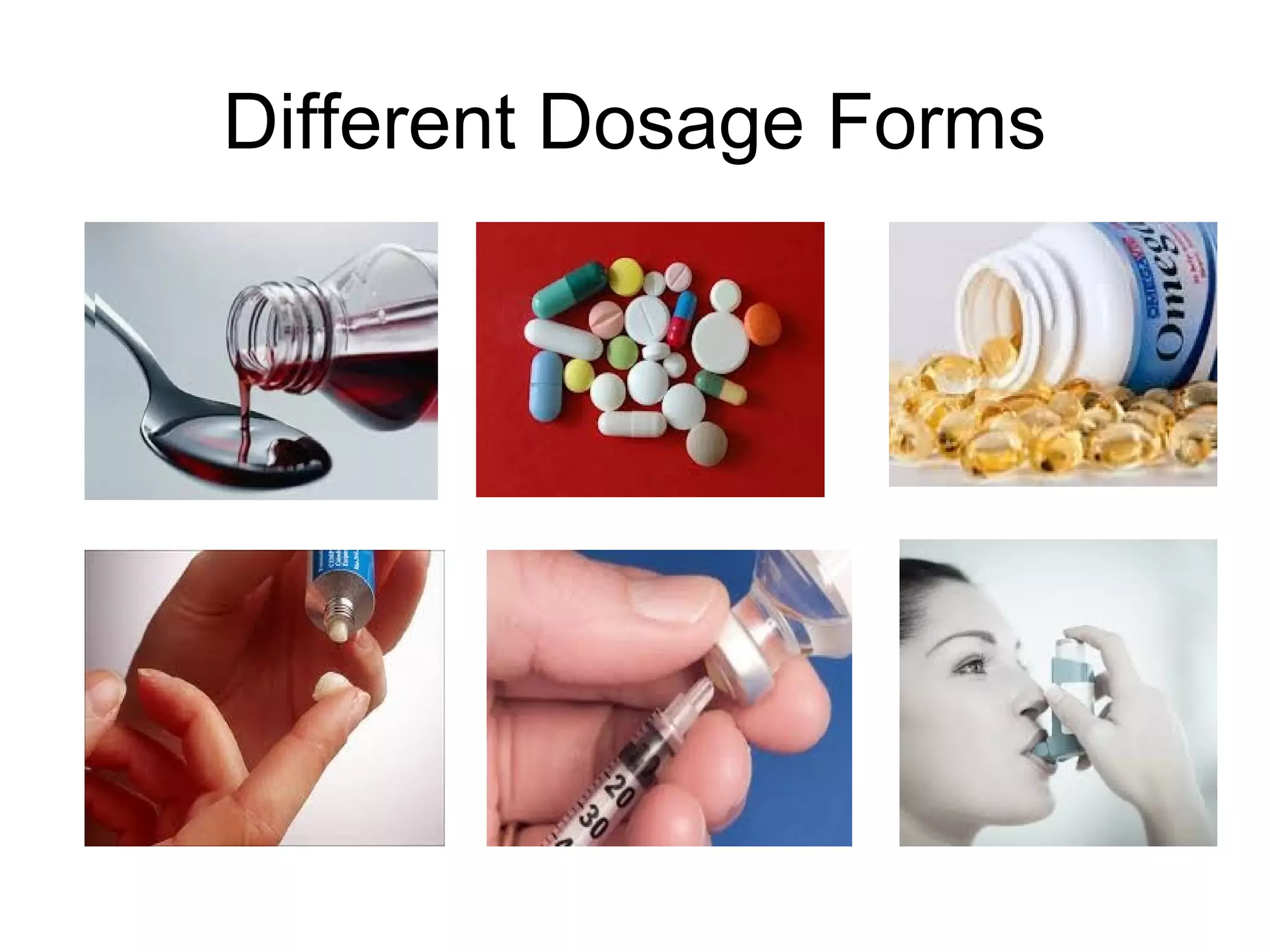
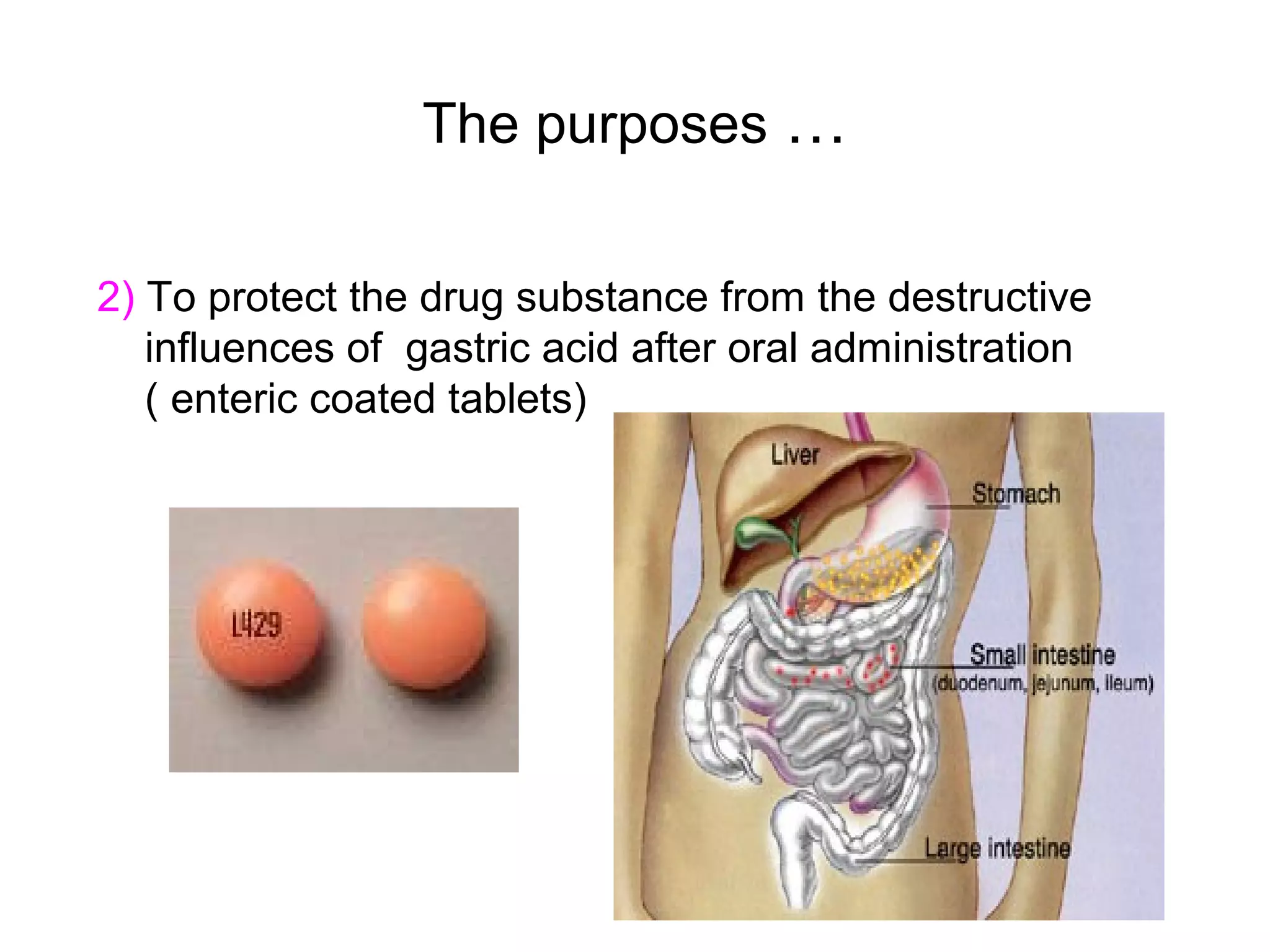
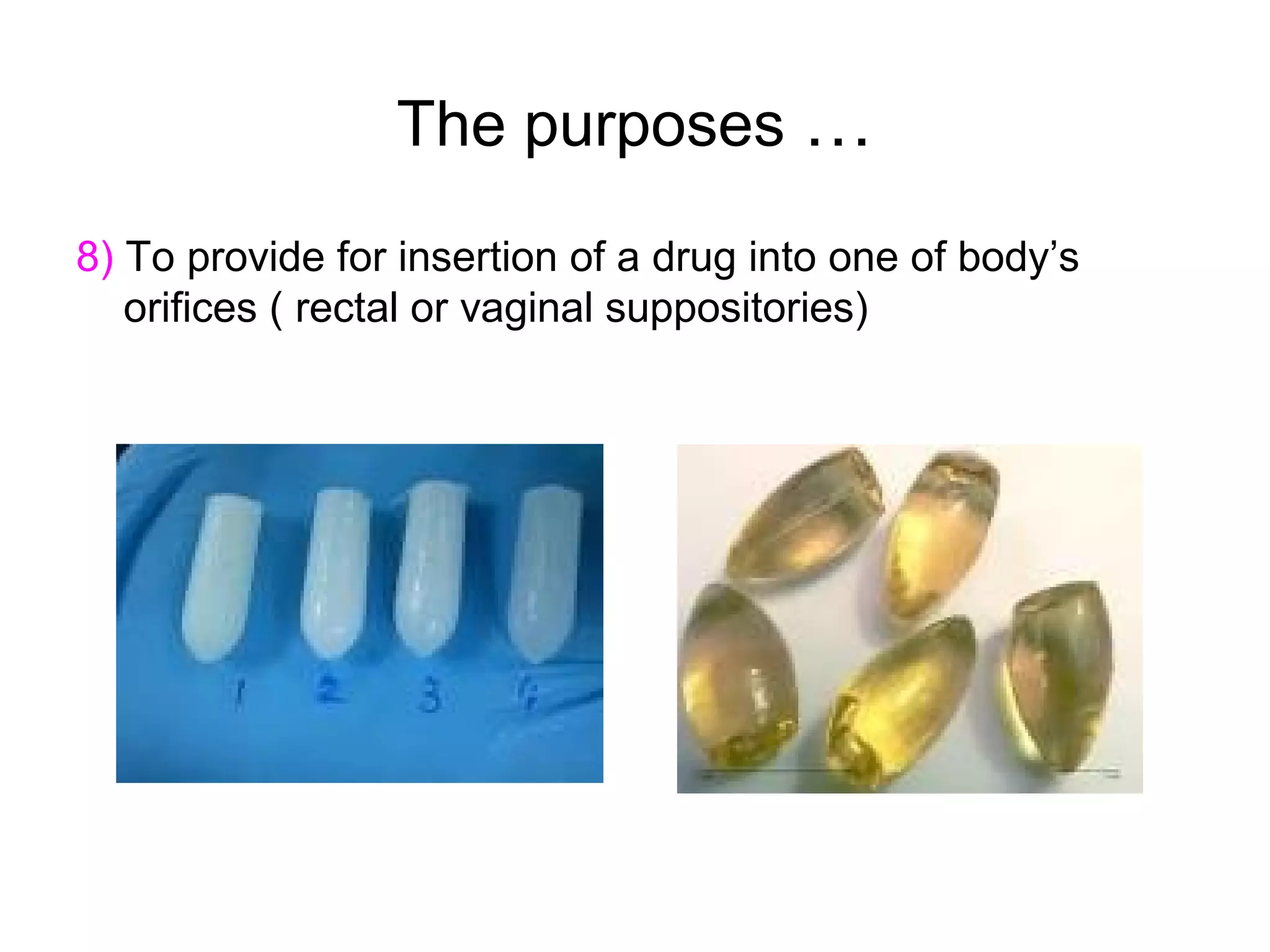
This document discusses pharmaceutical technology and considerations for developing drug dosage forms. Pharmaceutical technology deals with formulating new chemical entities into safe and effective medications for patients. Medicines contain drug substances formulated with excipients into dosage forms for delivery. Different dosage forms serve various purposes like protecting drugs, masking tastes, providing liquids or controlled release. Major considerations in designing dosage forms include physicochemical properties, biochemistry, and determining the optimal product type based on development goals. Therapeutic factors are also assessed like treatment type and patient attributes to select the appropriate dosage form.