Vitamin Solubility and Content Lab
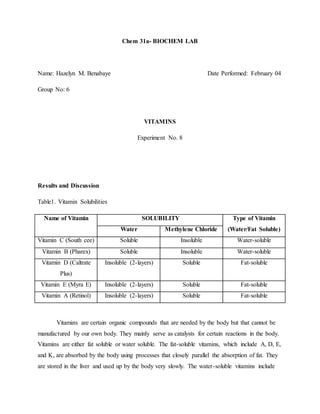
- 1. Chem 31a- BIOCHEM LAB Name: Hazelyn M. Benabaye Date Performed: February 04 Group No: 6 VITAMINS Experiment No. 8 Results and Discussion Table1. Vitamin Solubilities Name of Vitamin SOLUBILITY Type of Vitamin (Water/Fat Soluble)Water Methylene Chloride Vitamin C (South cee) Soluble Insoluble Water-soluble Vitamin B (Pharex) Soluble Insoluble Water-soluble Vitamin D (Caltrate Plus) Insoluble (2-layers) Soluble Fat-soluble Vitamin E (Myra E) Insoluble (2-layers) Soluble Fat-soluble Vitamin A (Retinol) Insoluble (2-layers) Soluble Fat-soluble Vitamins are certain organic compounds that are needed by the body but that cannot be manufactured by our own body. They mainly serve as catalysts for certain reactions in the body. Vitamins are either fat soluble or water soluble. The fat-soluble vitamins, which include A, D, E, and K, are absorbed by the body using processes that closely parallel the absorption of fat. They are stored in the liver and used up by the body very slowly. The water-soluble vitamins include
- 2. vitamin C and the B complex vitamins. The body uses these vitamins very quickly. Excess amounts are eliminated in urine. As shown in the table 2, water-soluble vitamins are soluble in water but are insoluble in water. On the other hand, fat-soluble vitamins are insoluble in water but soluble in methylene chloride. Like dissolves like, this means that water-soluble dissolves in water because they are both polar. On the other hand, fat-soluble vitamins dissolve in methylene chloride simply because fat-soluble vitamins are slightly an organic compound and methylene chloride is an organic compound. Table2. Standardization of Vitamin C B-1 117.7 g B-2 118 g B-3 191 mL B-4 191 mL CALCULATIONS B-5 300 mg B-6 190 mL B-7 1.58 mg/mL or 0.00158 g/mL Calculations 0.456 g → Vitamin C content Vitamin C is an antioxidant vitamin. It is useful in the body as a free-radical scavenger, that is, it reacts with any free radicals that it comes into contact with. A free radical is a molecule that has an odd number of electrons, and therefore is extremely reactive. Because they are so reactive, free radicals can actually cause damage to biological molecules such as DNA and proteins. Shown in table 2 are the calculations and values obtained from the experiment. Based on the reaction from the standardization of vitamin c, the vitamin c is oxidized and the iodine is
- 3. reduced to iodide ion. Starch was used as the indicator. Iodine formed a blue-black color complex with starch. Iodine was added on a solution that both contained vitamin C and starch. When the vitamin c was completely used up, the next drop of iodine reacted with starch, turning into a blue or gray solution. The appearance of this color was an indication that the vitamin C has been completely consumed. Using the formula mg vitamin C = mg vitamin C/1 mL iodine solution, thus, the value for mL iodine solution Vitamin C content in the solution is 0.456 g. Table3. Determination of Vitamin C in Fruit Juice Sample BURET READING VOLUME OF Iodine (mL) Mg Vitamin C Initial Final Oishi Sundae 0 6.4 6.4 0.01536- 1.536 % Oishi Sundae 6.4 13 6.6 0.01584- 1.584 % Oishi Sundae 13 22.8 9.8 0.02352- 2.352 % Vitamin C (ascorbic acid) is an antioxidant that is essential for human nutrition. Vitamin C deficiency can lead to a disease called scurvy, which is characterized by abnormalities in the bones and teeth. Many fruits and vegetables contain vitamin C, but cooking destroys the vitamin, so raw citrus fruits and their juices are the main source of ascorbic acid for most people. One way to determine the amount of vitamin C in food is to use a redox titration. Iodine is relatively insoluble, but this can be improved by complexing the iodine with iodide to form triiodide: I2 + I- ↔ I3 - Triiodide oxidizes vitamin C to form dehydroascorbic acid: C6H8O6 + I3 - + H2O → C6H6O6 + 3I- + 2H+ As long as vitamin C is present in the solution, the triiodide is converted to the iodide ion very quickly. However, when all the vitamin C is oxidized, iodine and triiodide will be present,
- 4. which react with starch to form a blue-black complex. The blue-black color is the endpoint of the titration. Using the formula, mL iodine solution x mg vitamin C = mg vitamin C in samples 1 mL iodine solution The following values shown in table 3 were obtained. Therefore, the amount of vitamin C in samplel 1 is 1.536%, for sample 2 is 1.584%, and for sample 3 is 2.352%. Calculations of mg Vitamin C in each sample If the daily requirement is 60 mg Vitamin C, how many milliliters (or grams) of each sample do you need to need the minimum daily requirement? Table4. Heat destruction of vitamin C Heating Time BURET READING Volume Iodine mg Vitamin C mg Vitamin C lostInitial Final 10 23 mL 38.2 mL 15.2 mL 36.48 mg 963.52 mg Heating can destroy the amount of vitamin C in the food. Raw citrus fruits and their juices are the main source of ascorbic acid for most people. Based on the experiment, small amount of iodine was added to the sample through titration until the iodine that was added no longer disappears. This is due to the amount of iodine added to the sample was greater than the amount of vitamin C that was there, so all the vitamin C was destroyed and there were some
- 5. iodine that were left over. In this experiment, the role of starch was to pair up with iodine in order to obtain a dark-blue color. As shown in table 4, the solution was heated for only 10 minutes and the amount of vitamin C lost was 963.52 mg. This proves that vitamin C is easily oxidized when heated. Heating fruit juice loses vitamin C content. It is said that the higher the temperature, the vitamin C will be easily damaged as heat will cause the rapid movement of molecules and that vitamin C is a water-soluble vitamin and water-soluble vitamins are easily destroyed by heat. Vitamin C in Urine Conclusion Vitamins are certain organic compounds that are needed by the body but that cannot be manufactured by our own body. They mainly serve as catalysts for certain reactions in the body. Vitamins are either fat soluble or water soluble. The two classifications of vitamins include the water-soluble and fat soluble vitamins. The fat-soluble vitamins, which include A, D, E, and K, are absorbed by the body using processes that closely parallel the absorption of fat. They are stored in the liver and used up by the body very slowly. The water-soluble vitamins include vitamin C and the B complex vitamins. Vitamin C content in a variety of citrus juices and other solutions were determined through the tests that were conducted. And also, it was proved that heating such food or fruit juices loses vitamin C because they are easily damaged through exposure of high temperature. This experiment is important for those who suffer scurvy, a disease caused by insufficiency of vitamin C in the body, to avoid heating such foods in order to avoid such disease.