Embed presentation










The Trial Master File (TMF) is a compilation of essential documents that demonstrate compliance with study protocols and quality data management during clinical trials. It encompasses both sponsor and investigator files, necessitating segregation of documents generated by each party. Responsibilities for TMF maintenance and management are often delegated to research team members or subcontracted to Clinical Research Organizations (CROs), ensuring adherence to guidelines and standard operating procedures.












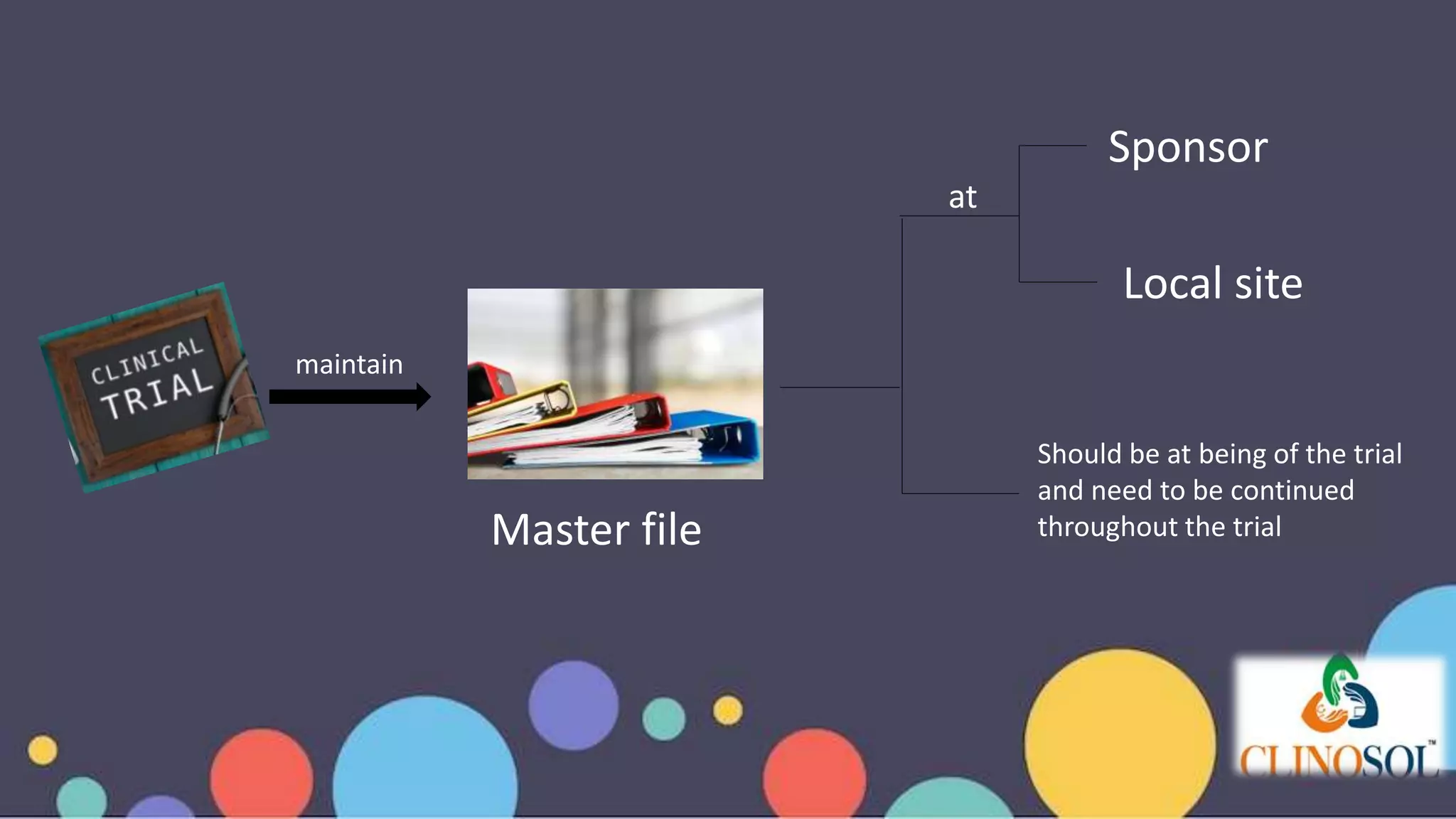
Introduction to Trial Master File (TMF) as a collection of documents for trial conduct and compliance.
Emphasis on data quality during trials and ongoing maintenance of TMF at sponsor and site level.
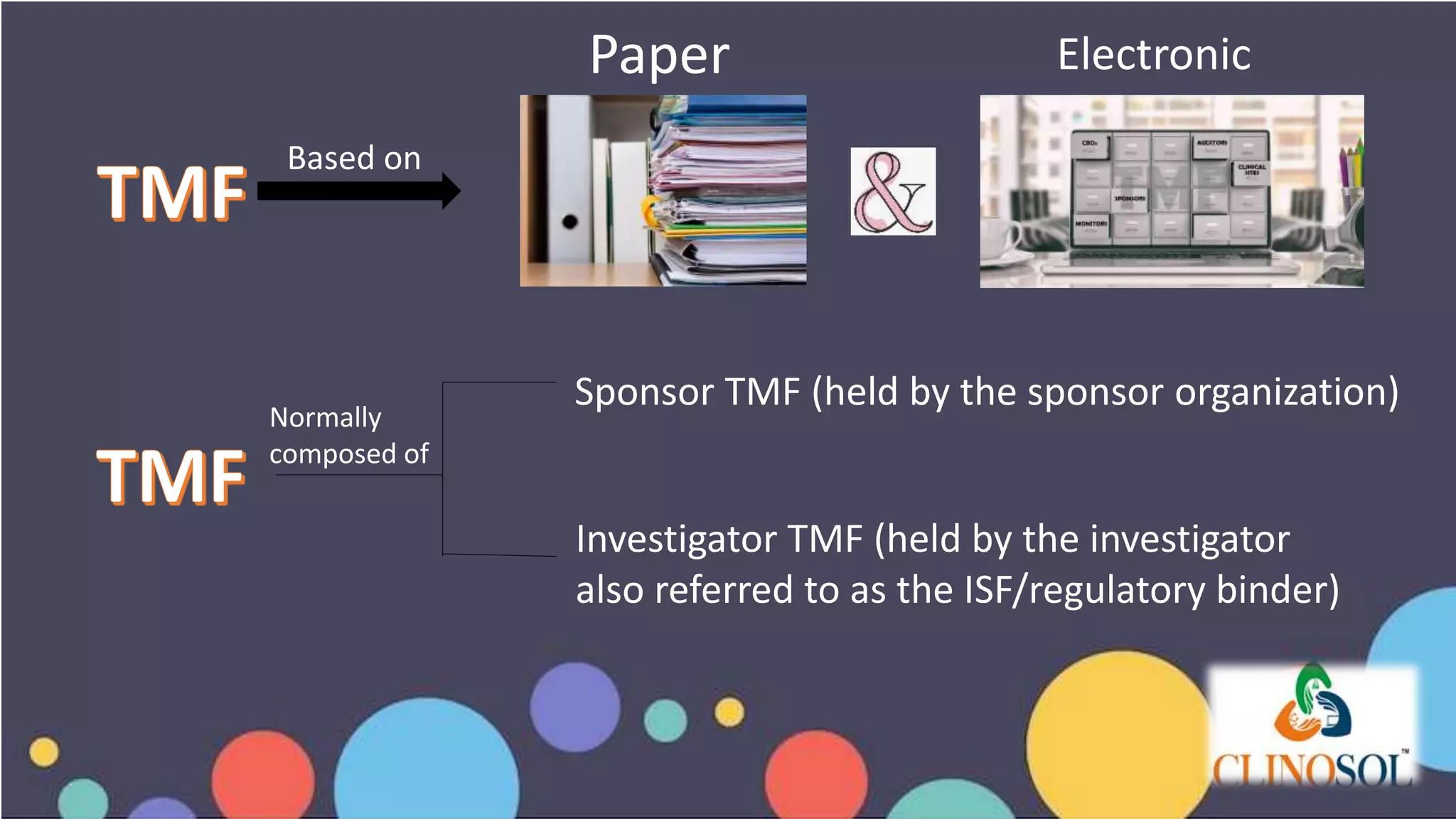
Distinction between Sponsor TMF and Investigator TMF, and examples of documents managed by each.
Overview of essential documents and standardized processes for TMF as outlined by ICH guidelines.
Responsibilities for TMF updates and quality control, including delegation and CRO involvement.
Sources for further reading on TMF alongside contact details for inquiries.