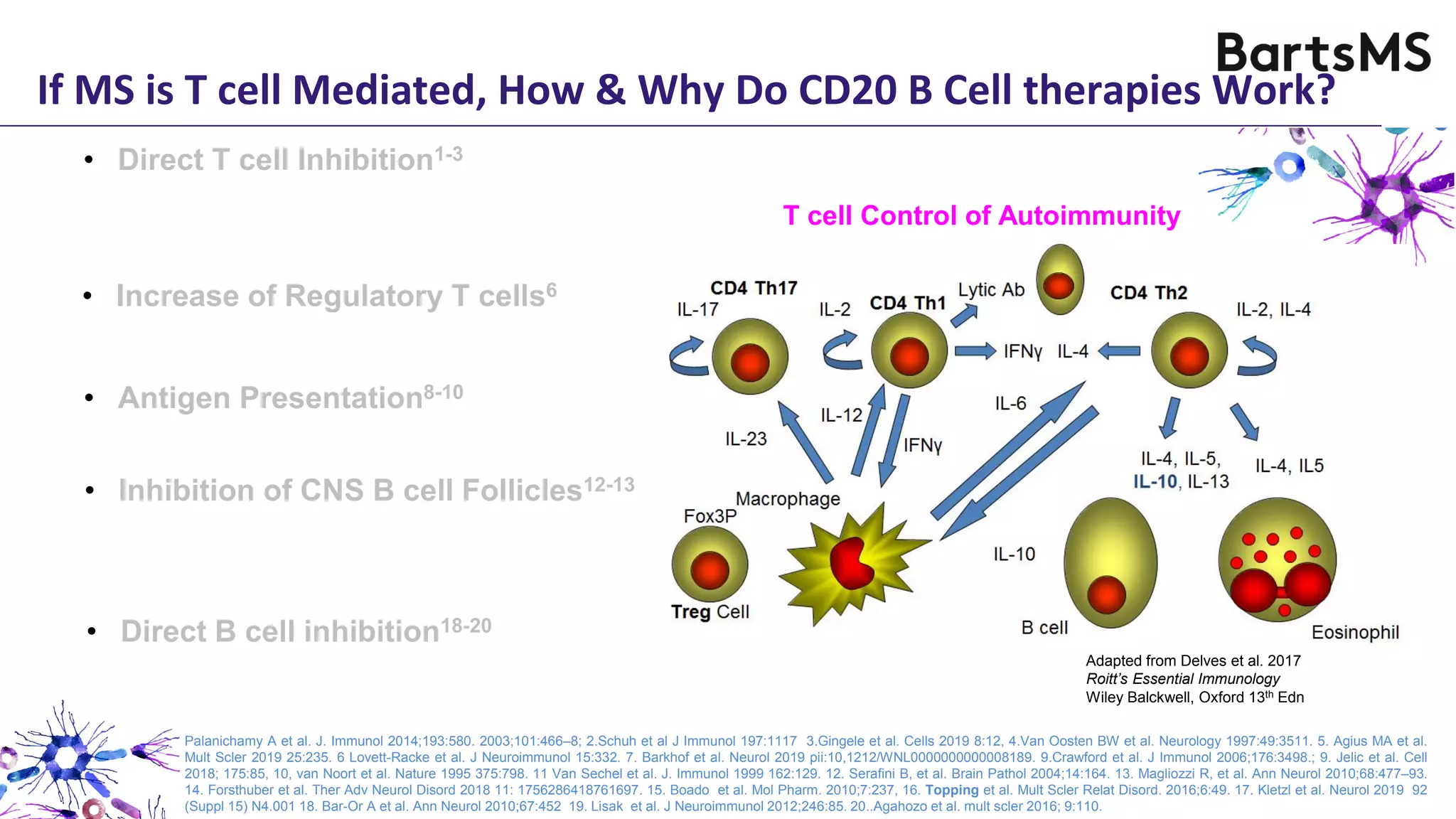
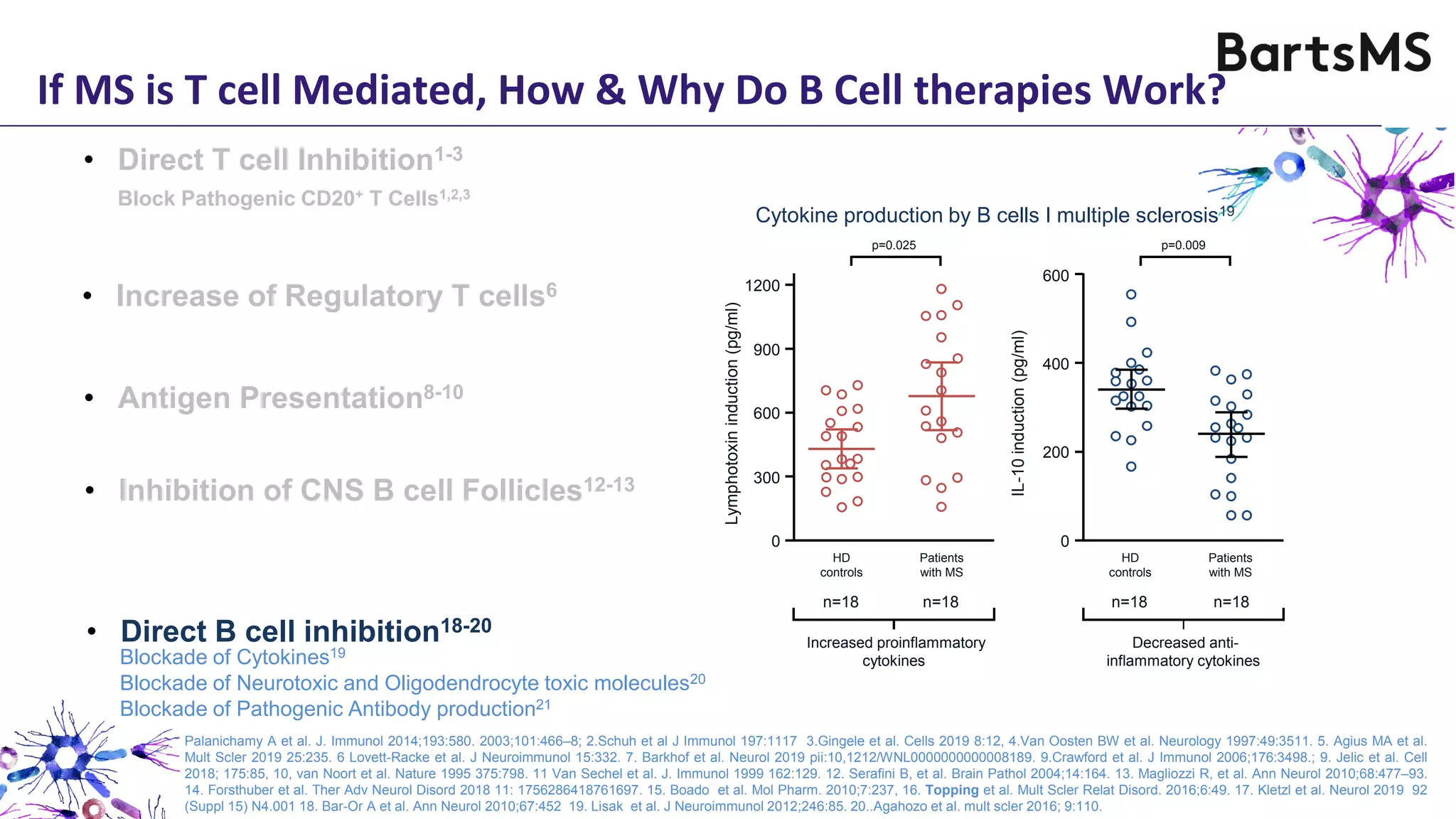
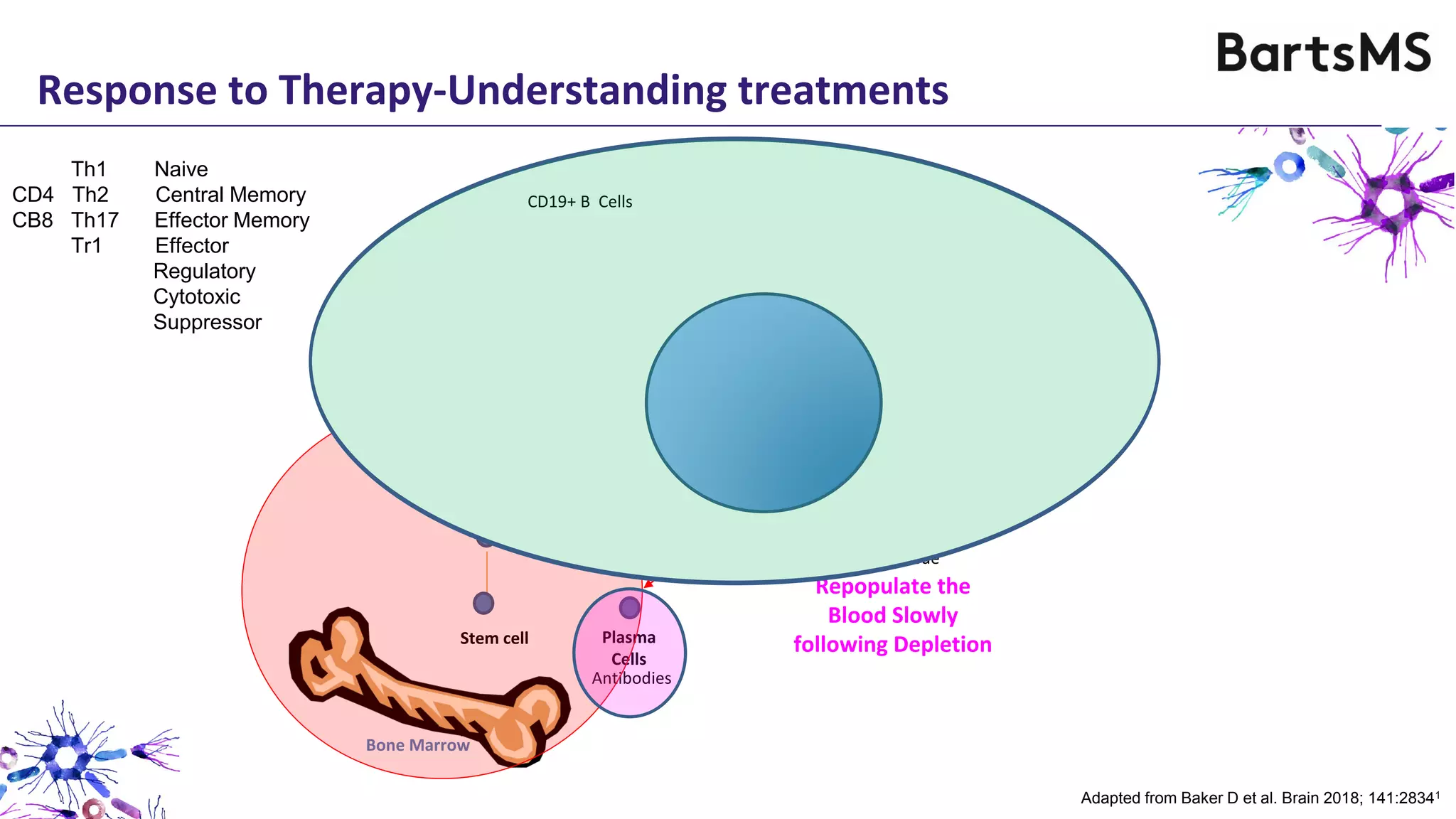
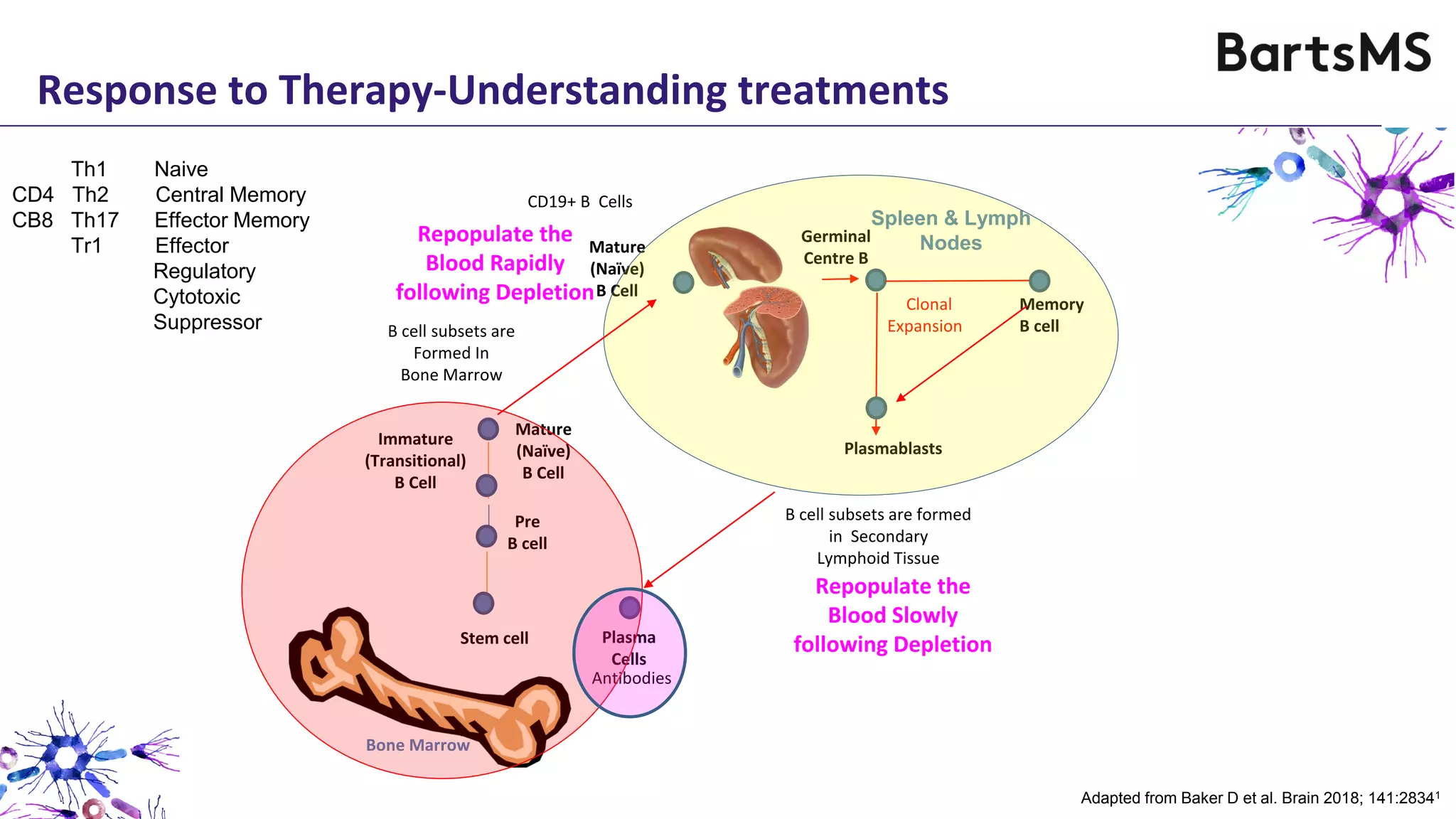
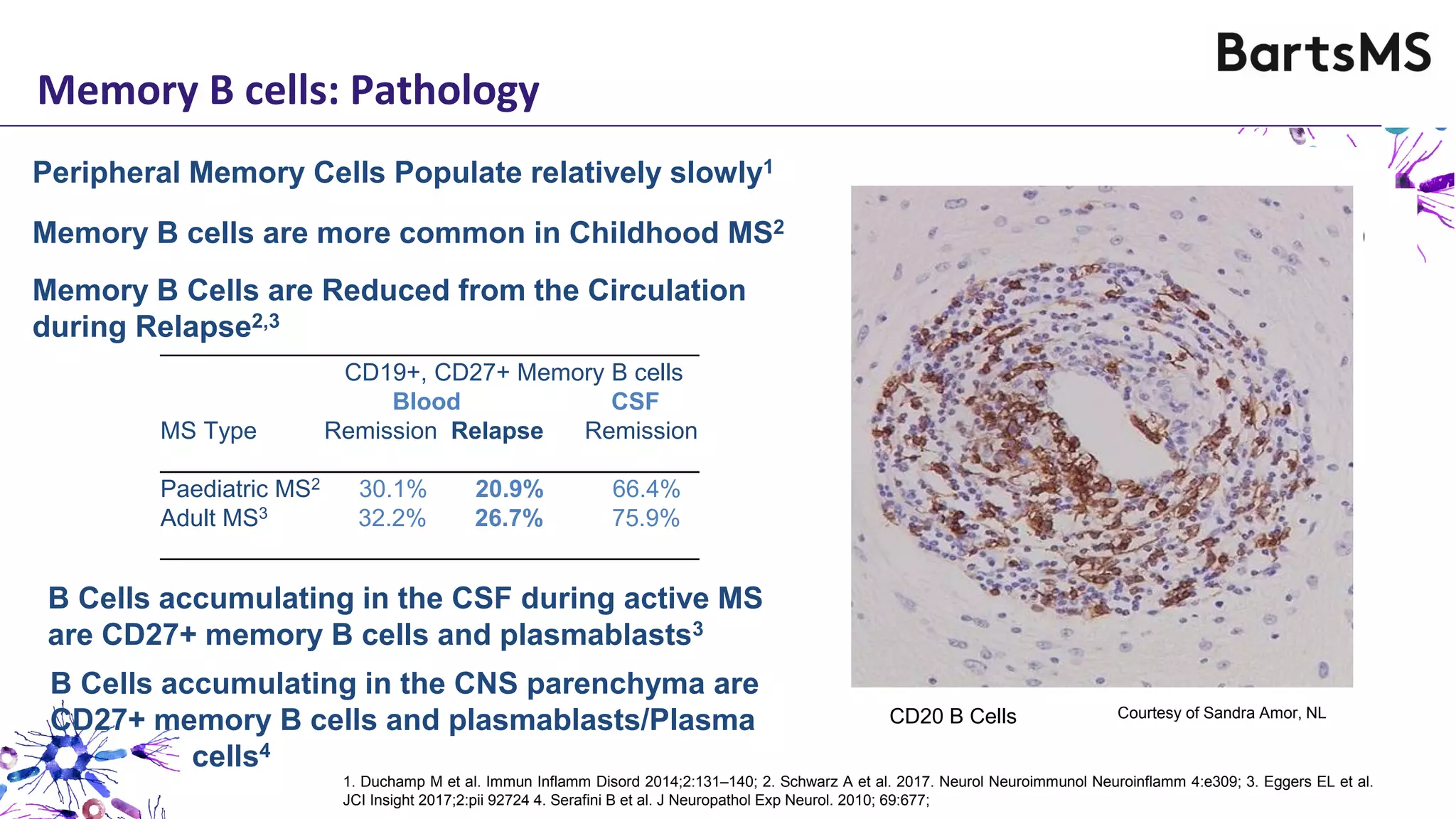
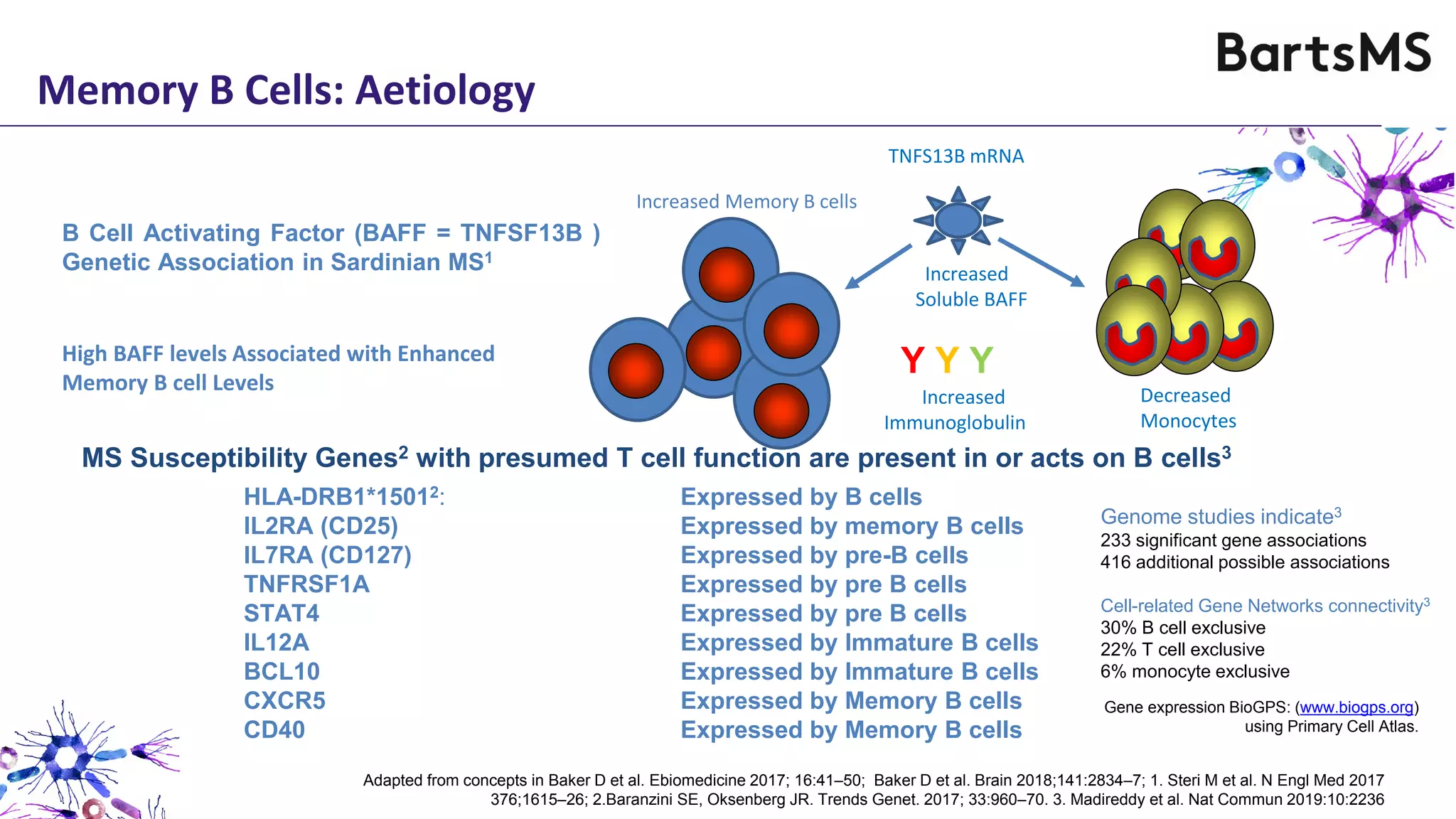
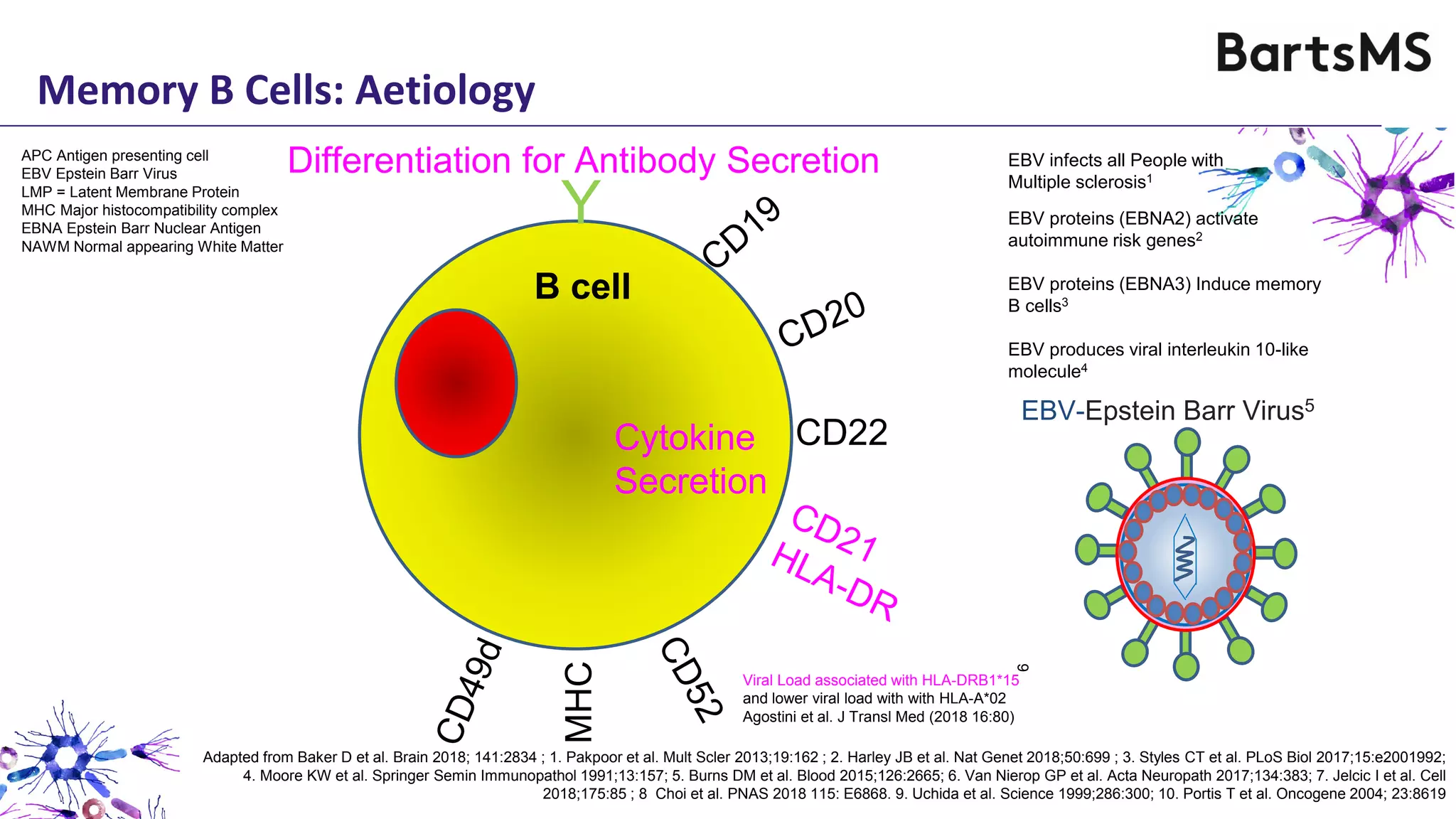
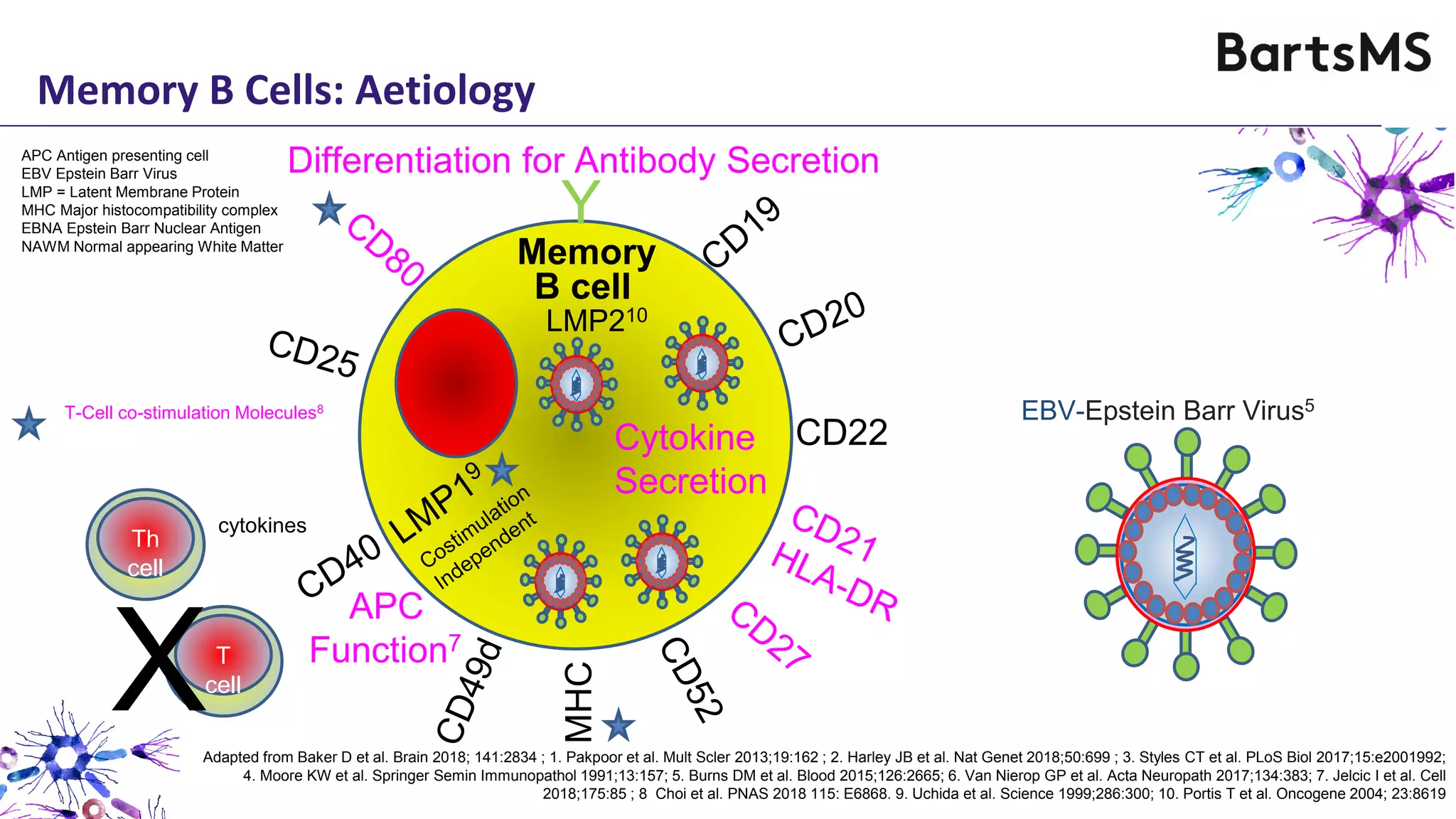
- CD20 B cell therapies like ocrelizumab work in multiple sclerosis through several mechanisms including direct inhibition of T cells, increasing regulatory T cells, blocking antigen presentation, and directly inhibiting B cells. While MS is considered a T cell-mediated disease, B cells can induce the proliferation and activation of pathogenic T cells. CD20 therapies may work by depleting this pathogenic B cell population. However, the mechanisms are not fully understood as CD20 therapies do not fully deplete long-lived plasma cells in the central nervous system.