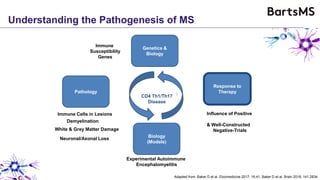
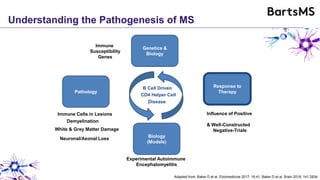
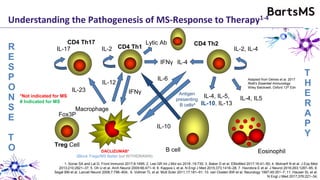
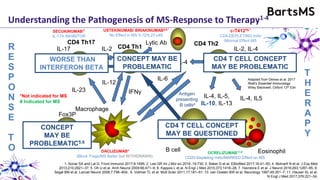
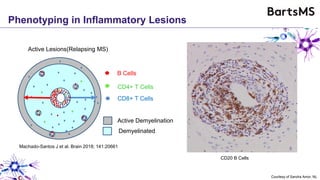
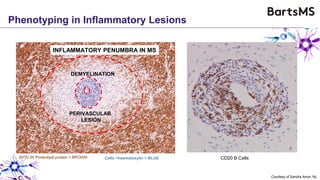
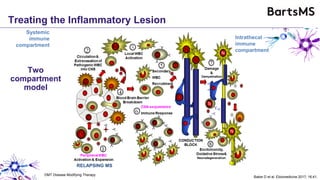
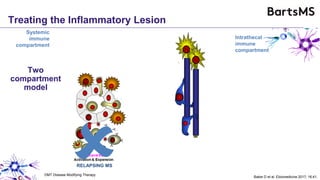
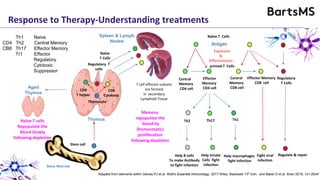
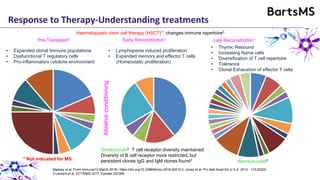
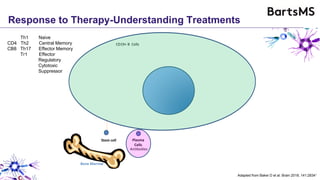
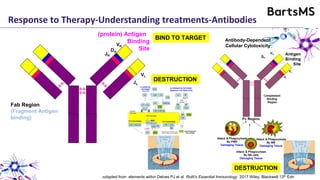
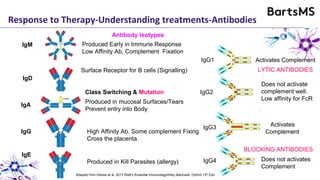
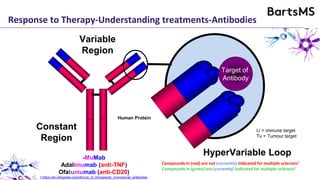
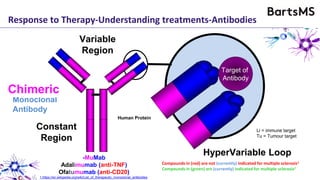
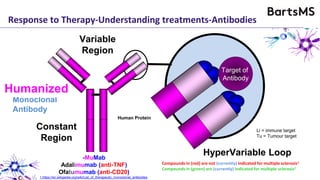
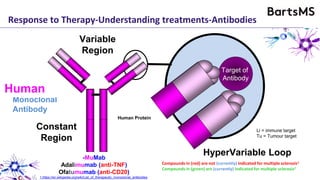
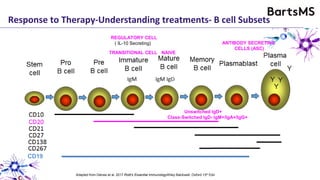
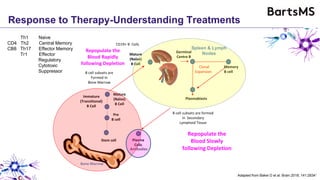
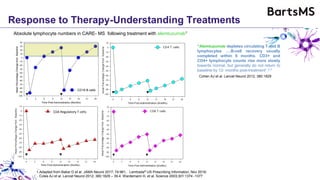
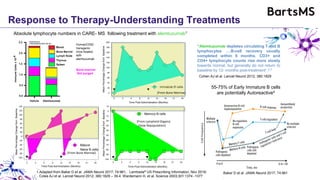
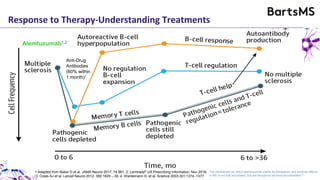
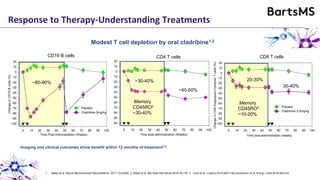
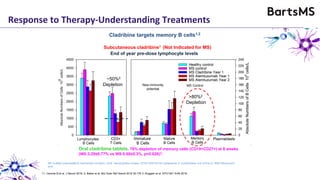
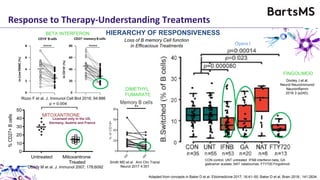
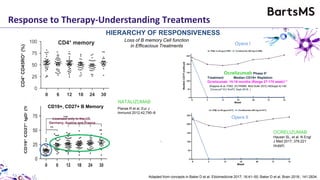
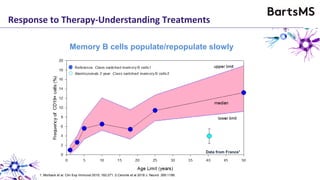
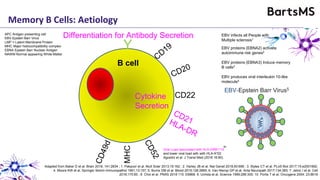
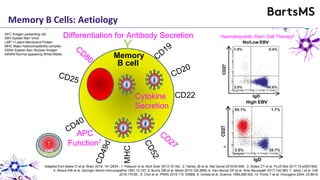
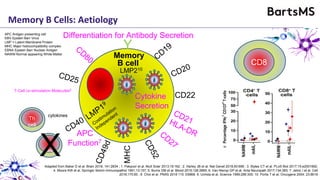
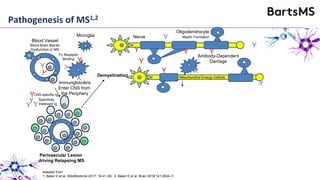
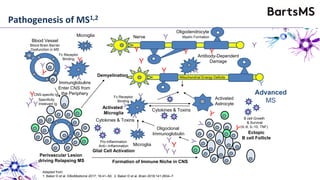
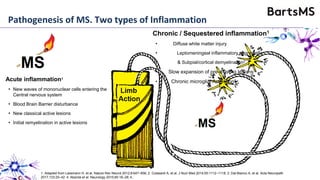
This document summarizes a presentation given by Professor David Baker on B cell therapy for multiple sclerosis. It discusses B cell subsets, the role of B cells in the pathogenesis of MS, response to different therapies as a key experiment to understand biology, and repopulation characteristics of immune cells after treatment. It also provides diagrams on immune cell differentiation and repopulation, the two compartment model of treating inflammatory lesions in MS, phenotypes seen in active MS lesions, and types of monoclonal antibodies used as treatments.