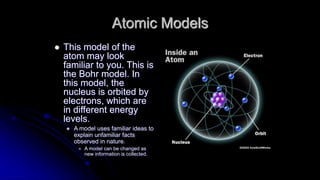
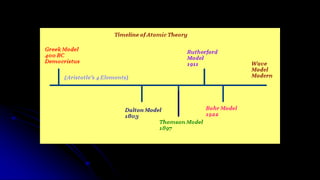
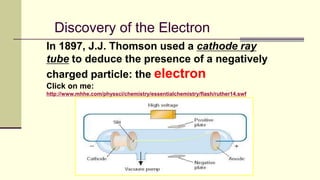
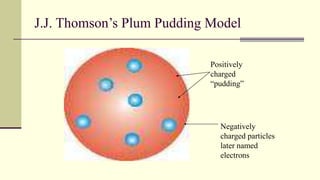
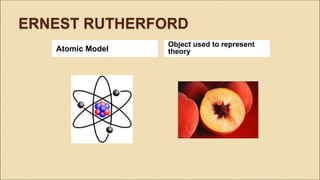
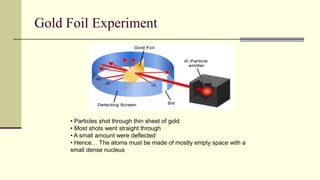
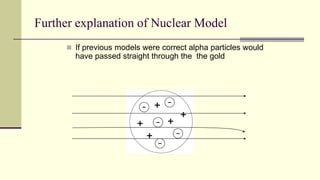
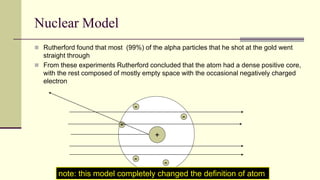
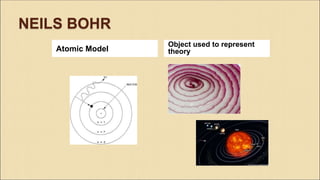
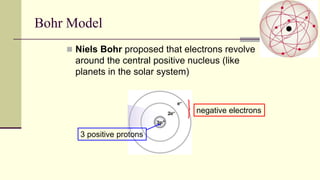
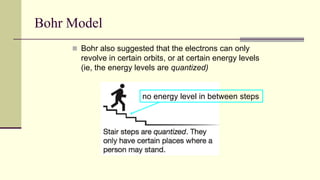
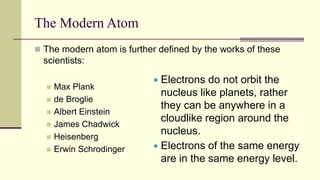
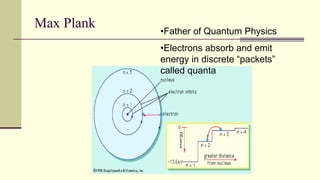
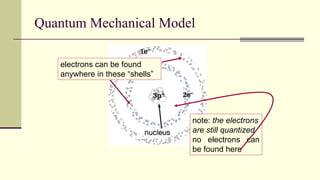
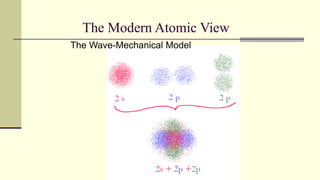
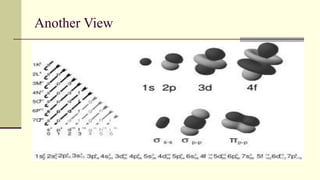
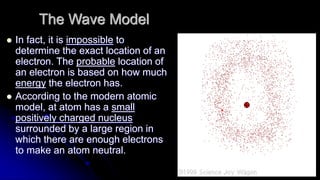
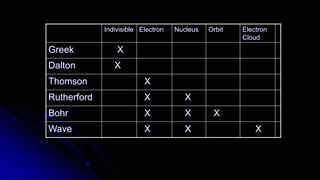
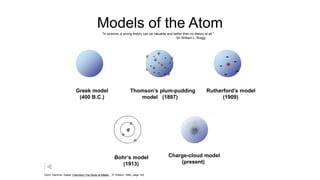
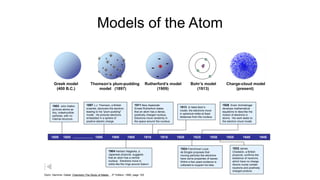
Democritus first proposed in 400 BC that matter is made of indivisible particles called atoms. This idea was ignored for over 2000 years. In the early 1800s, Dalton proposed that atoms are small, hard spheres that make up elements. Thomson's 1897 discovery of the electron led to his "plum pudding" model of atoms with positive charge and embedded electrons. Rutherford's 1909 gold foil experiment showed that atoms have a small, dense nucleus surrounded by empty space. Bohr's 1913 model depicted electrons orbiting the nucleus in fixed energy levels like planets around the sun. Modern atomic theory describes electrons as existing in probabilistic "clouds" or orbitals around the nucleus based on quantum mechanics.