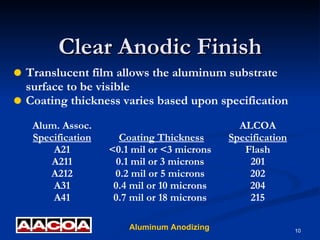
Anodizing is an electrochemical process that converts the metal surface of aluminum to aluminum oxide. It produces a coating that is very durable, corrosion resistant, and maintains the metallic appearance of the aluminum. The anodizing process involves racking parts for processing, cleaning, etching, anodizing in an acid bath using electricity, coloring or sealing the pores, and testing to quality check the coating. Anodized aluminum has advantages like durability, low maintenance, and an environmentally friendly process.