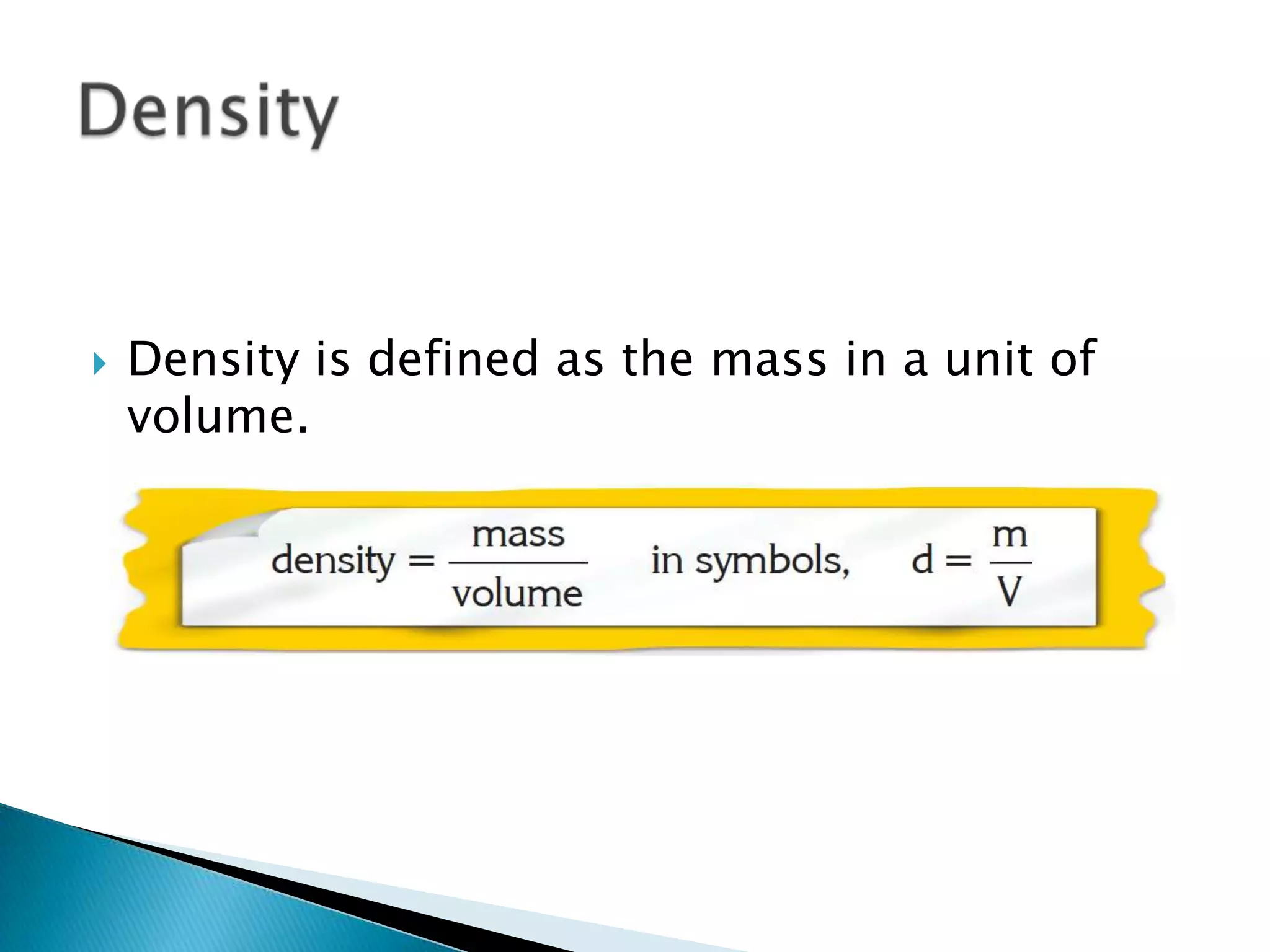
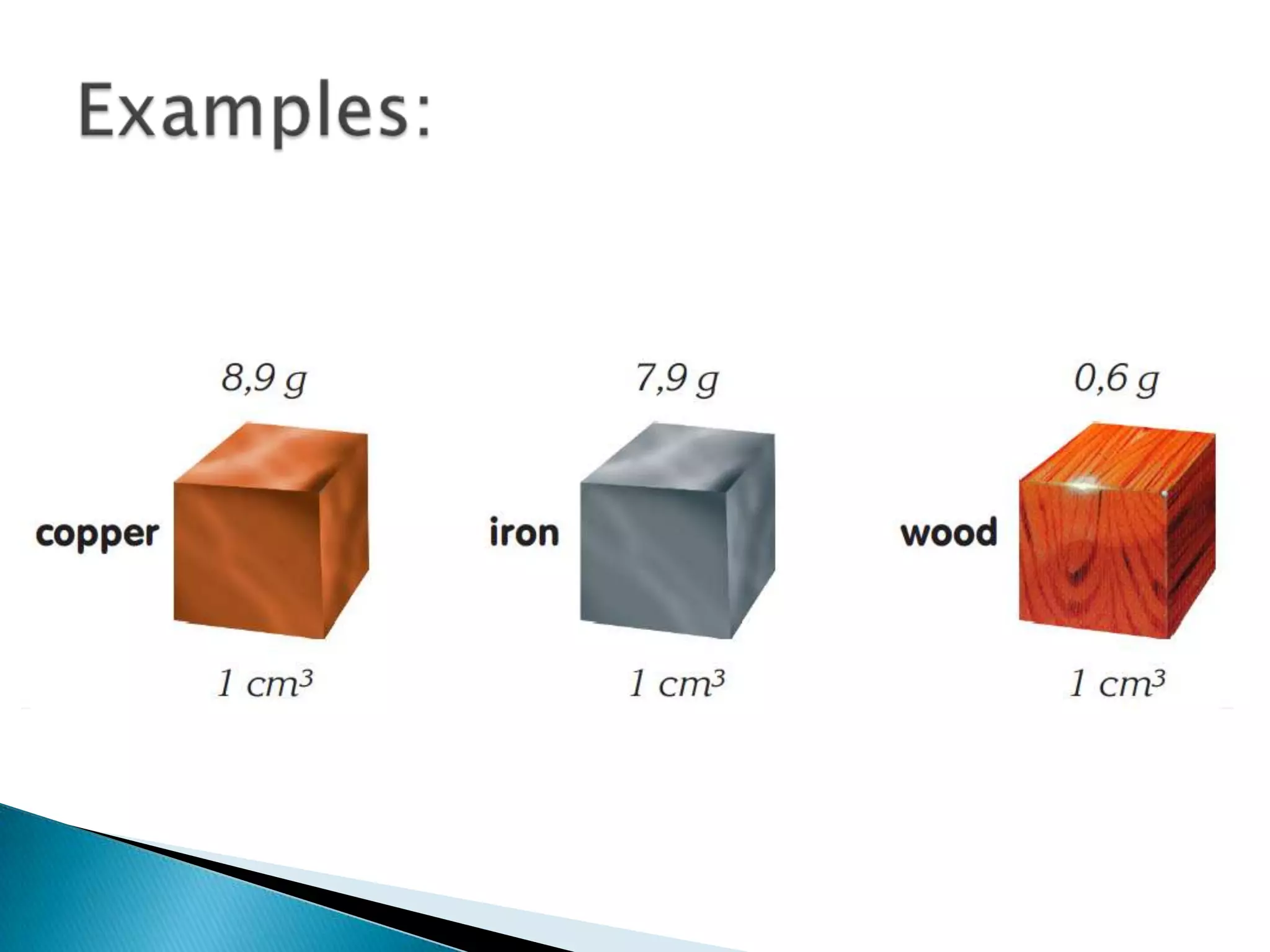
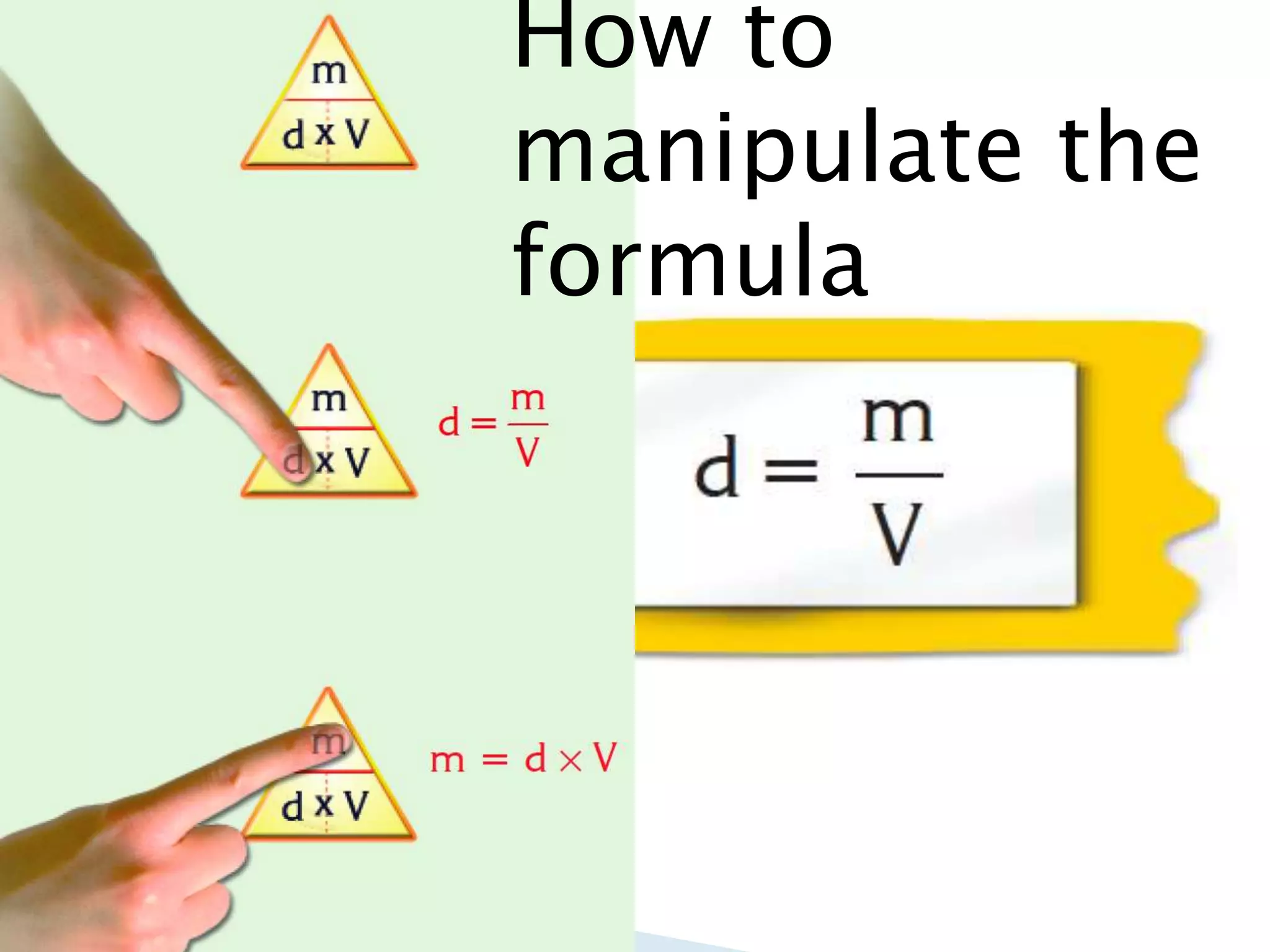
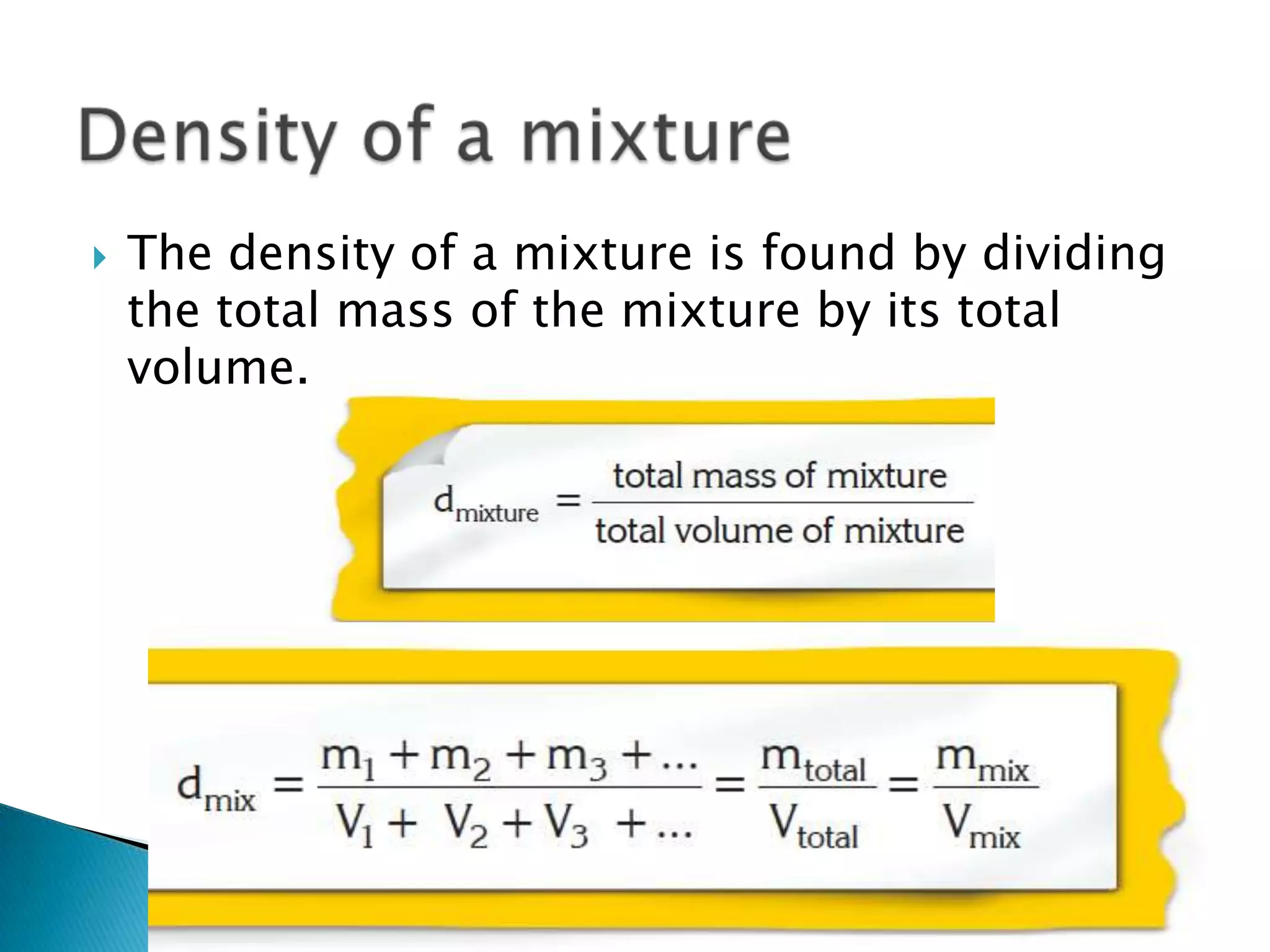
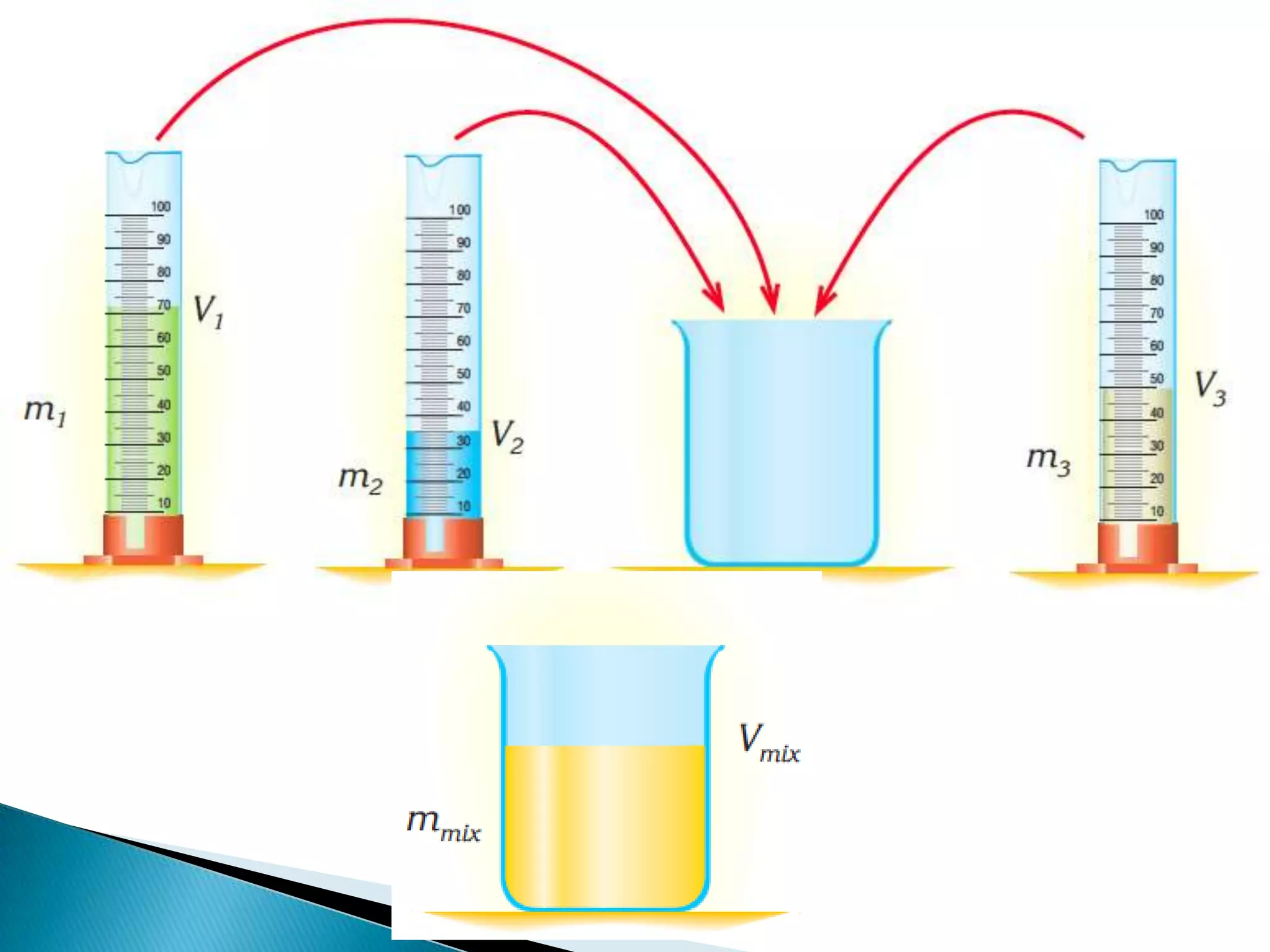
Density is defined as mass per unit volume and is commonly measured in grams per cubic centimeter. The document provides examples of how to calculate density by dividing an object's mass by its volume. It also gives examples of density calculations and determining the density of mixtures.