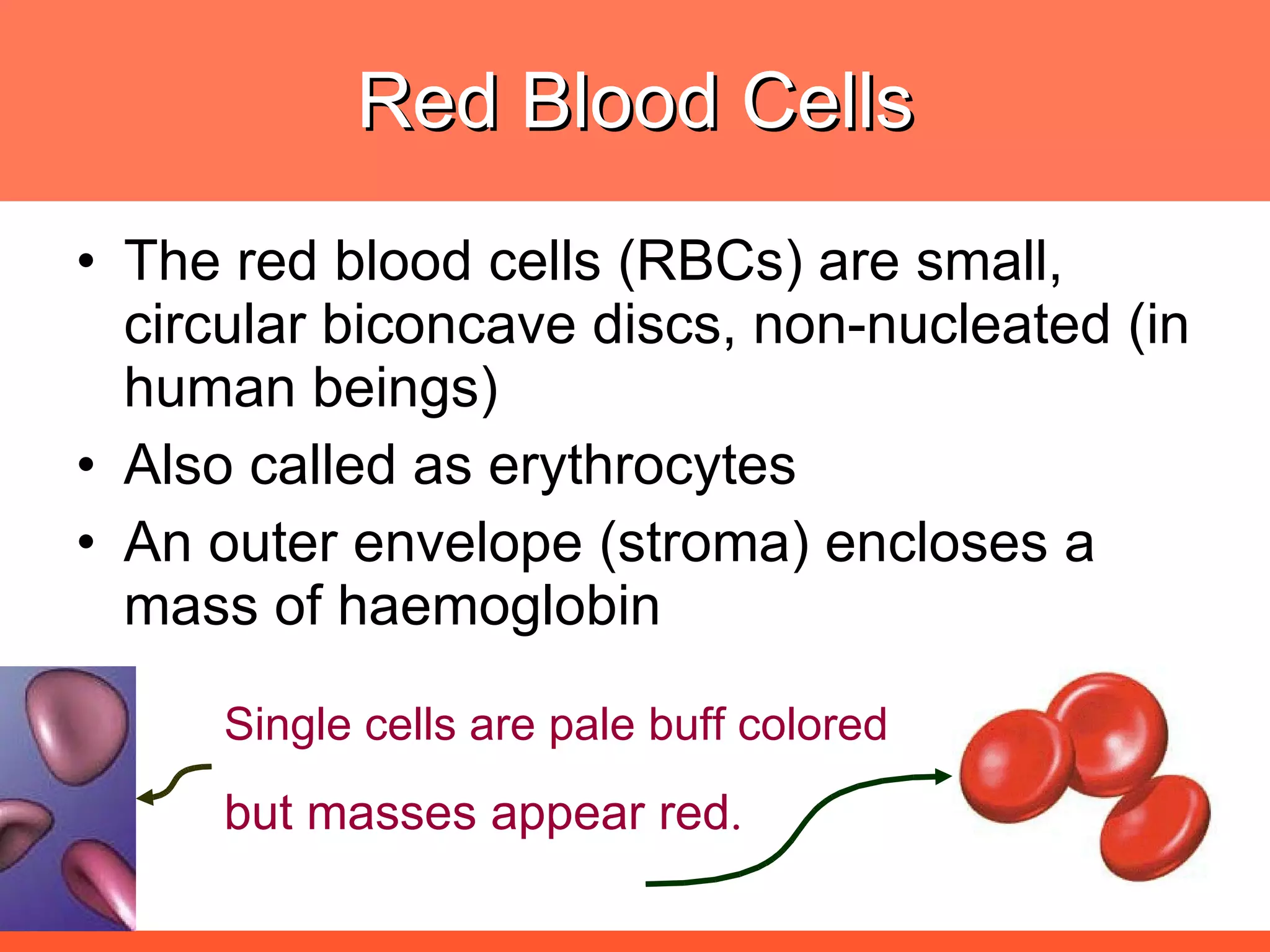
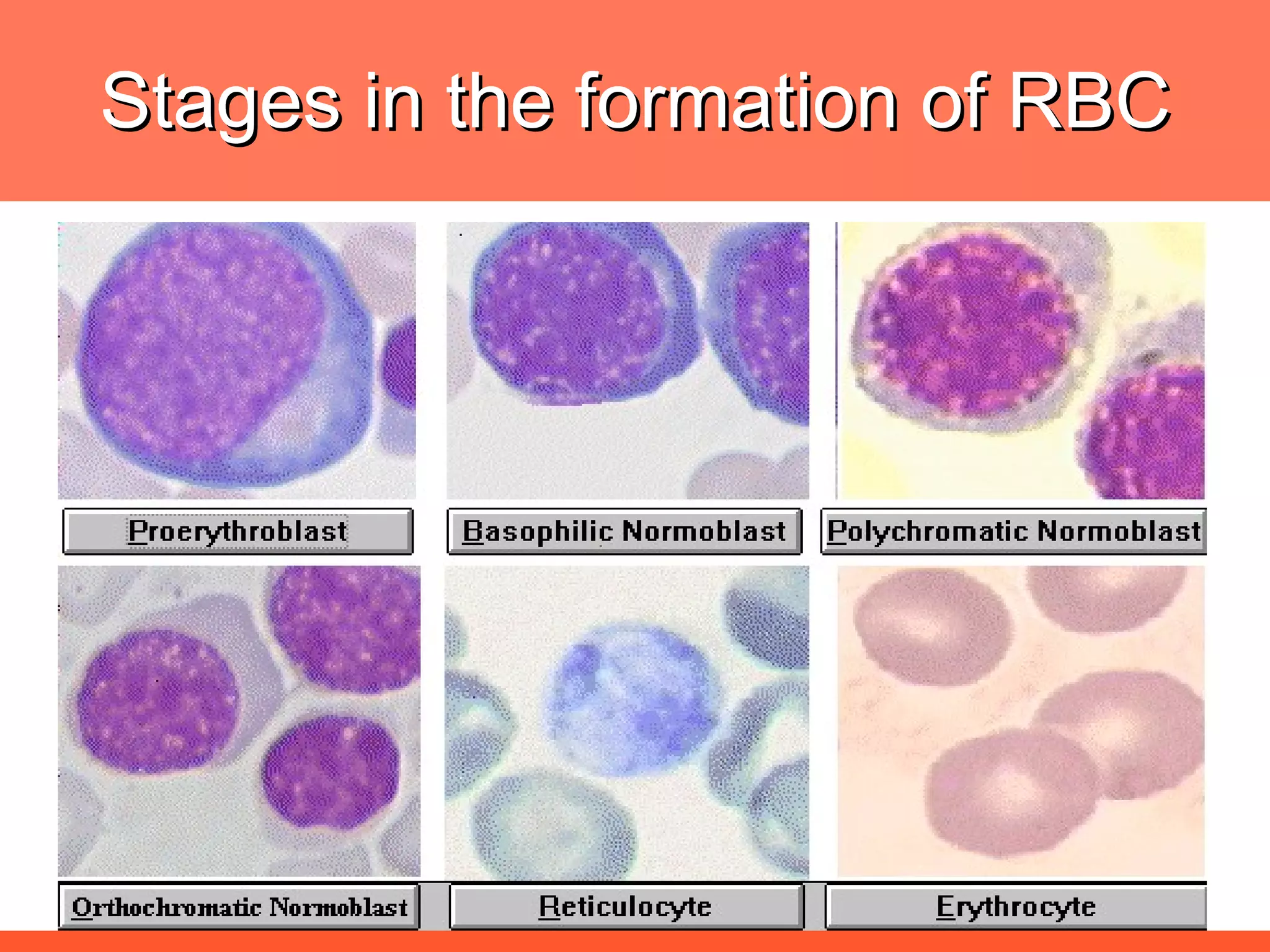
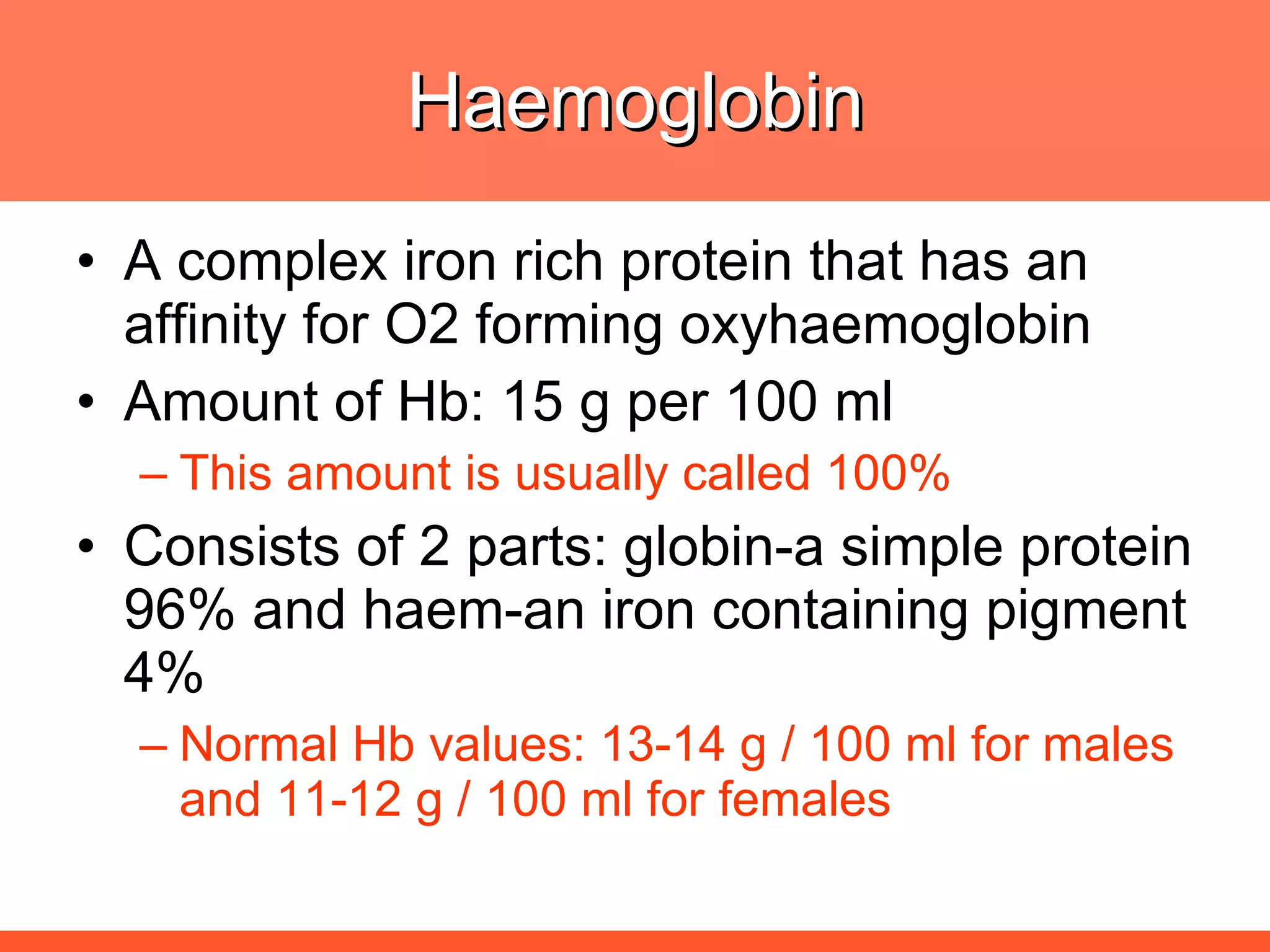
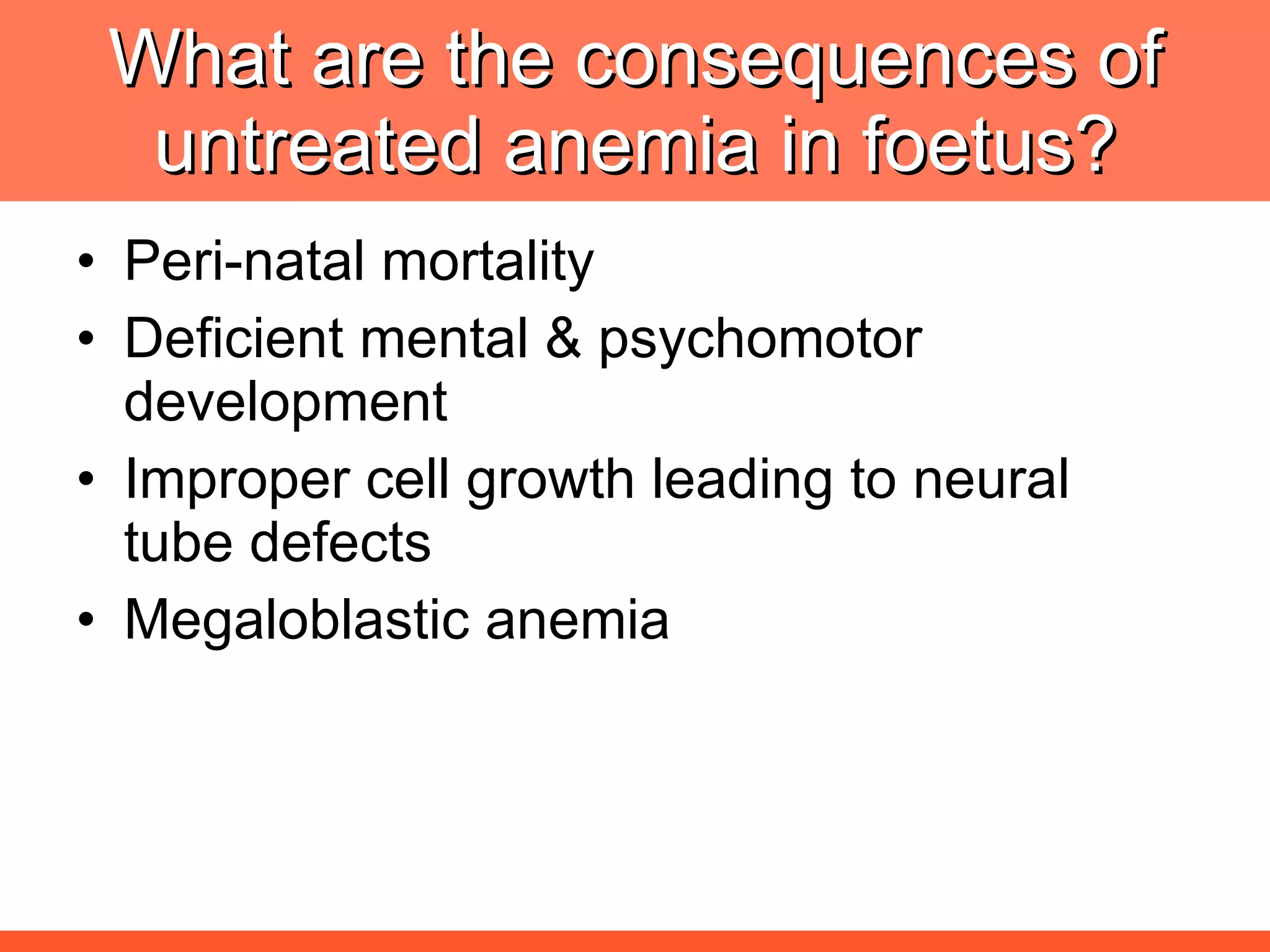
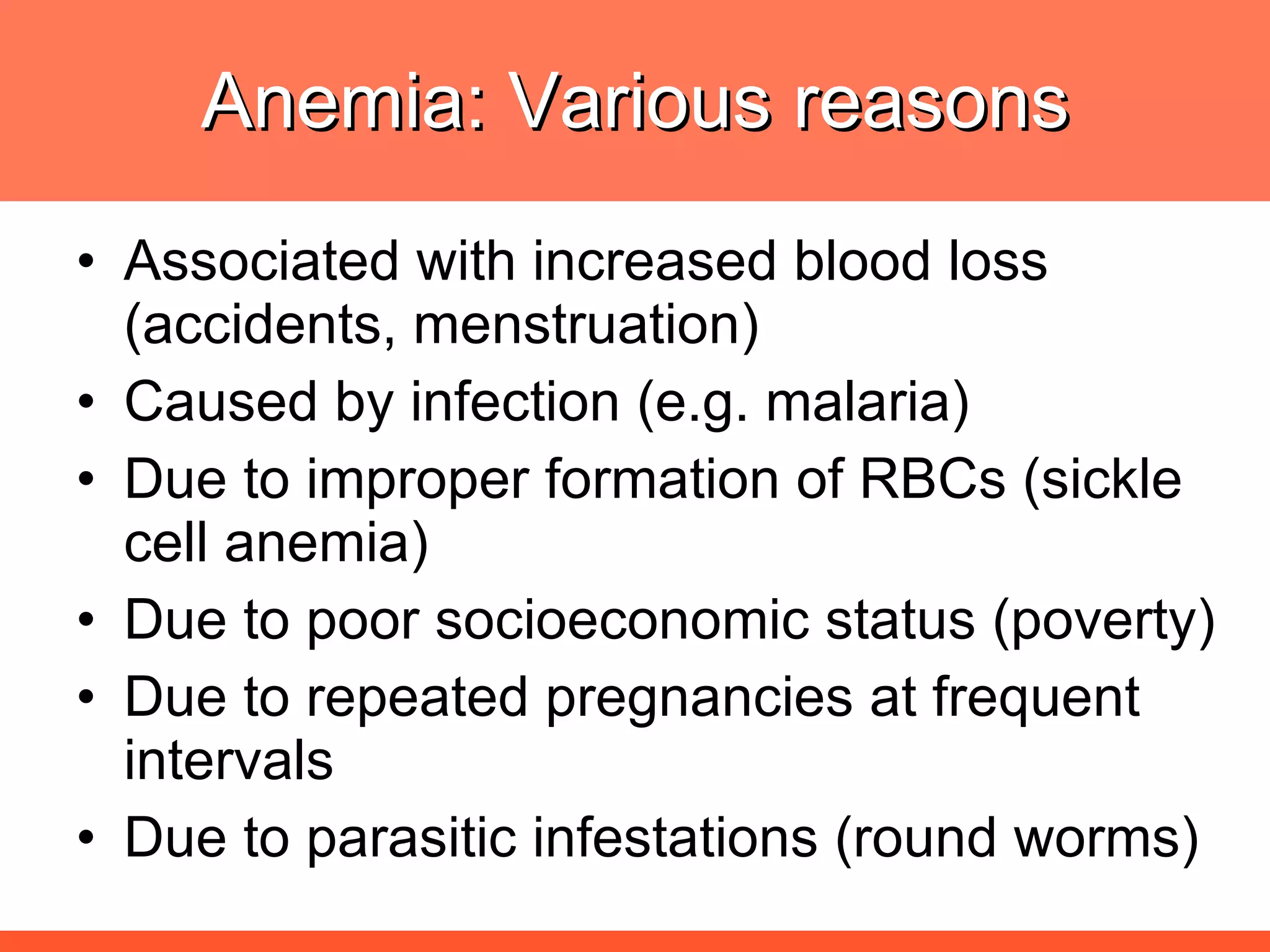
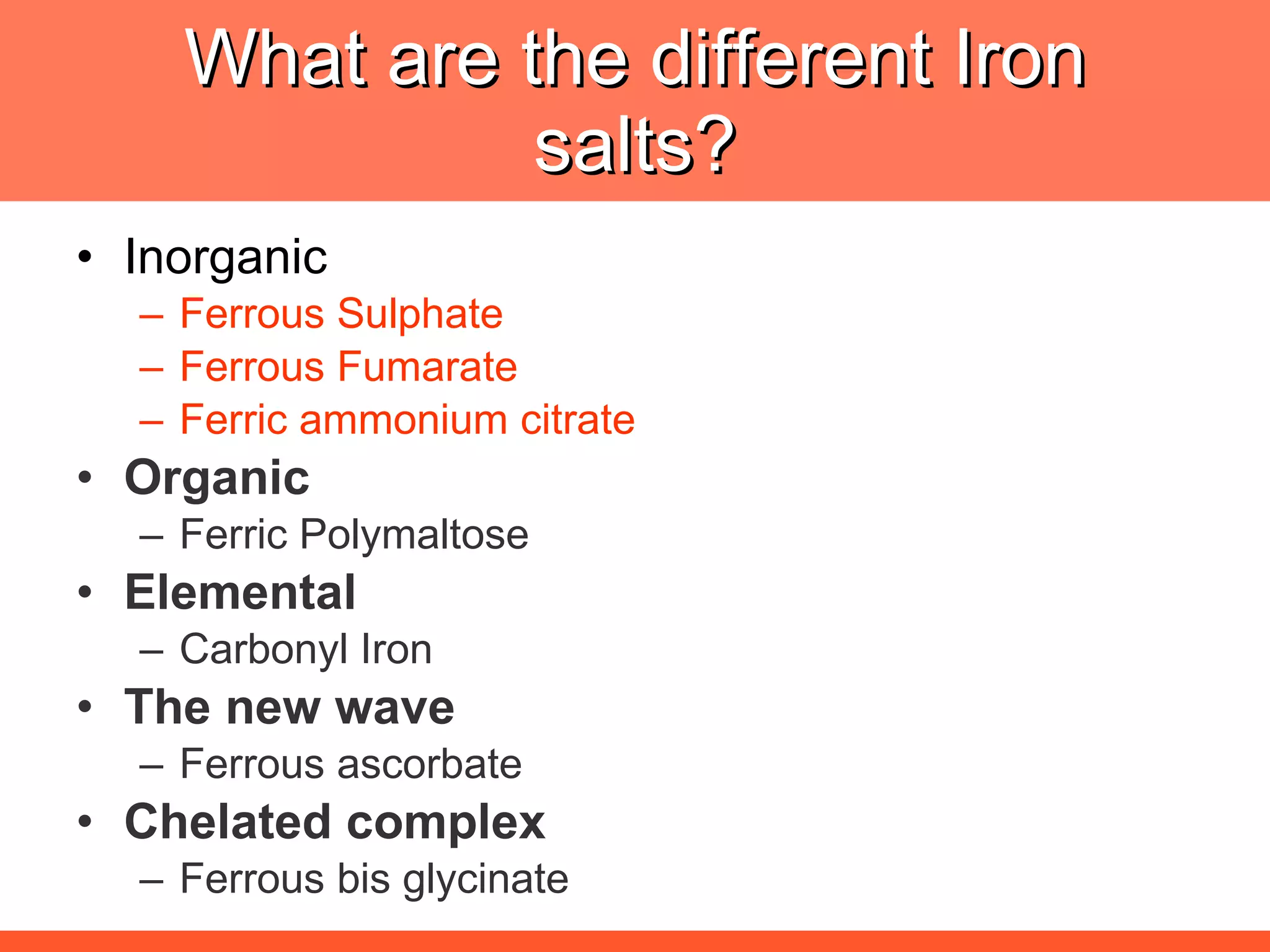
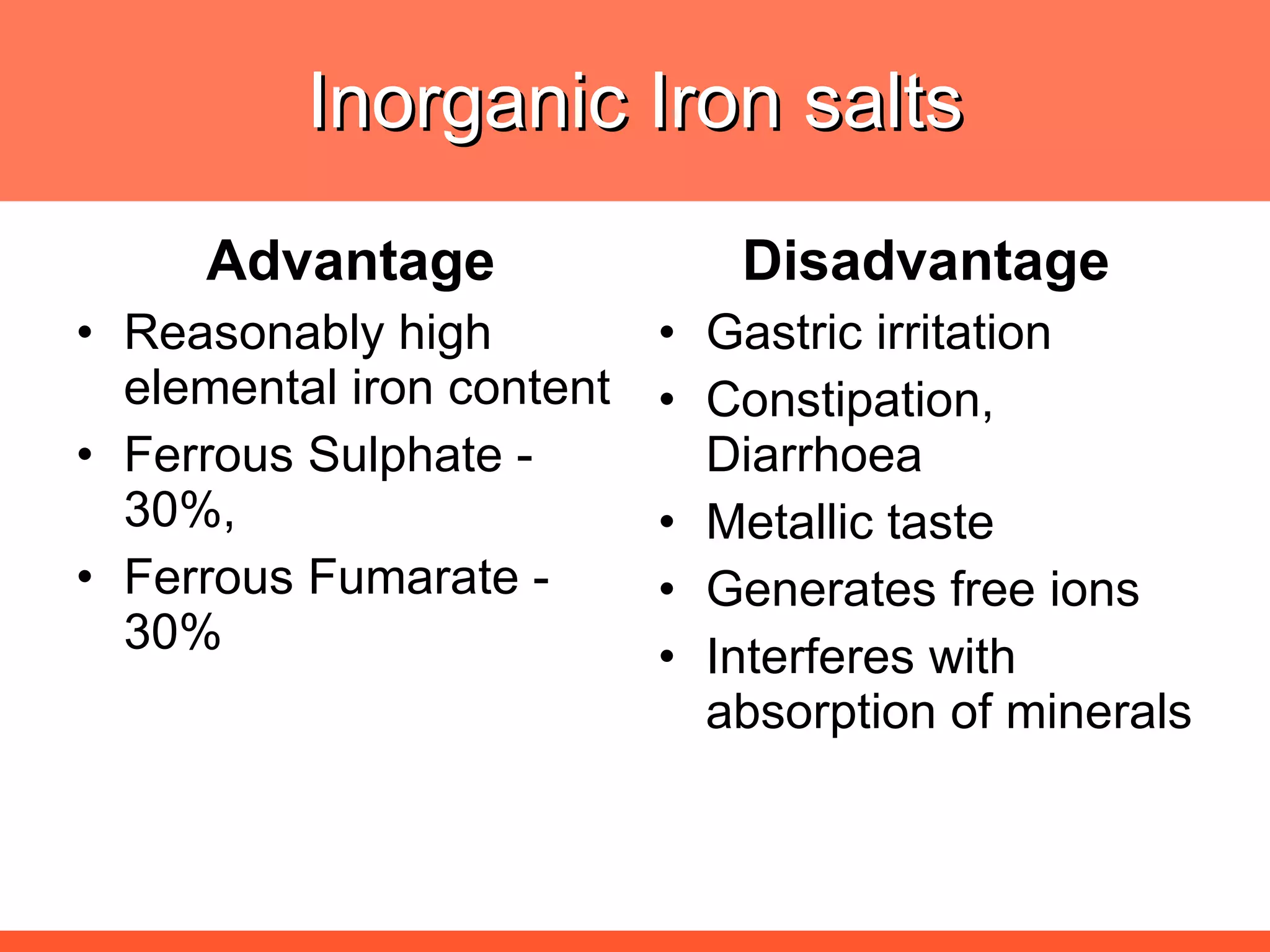
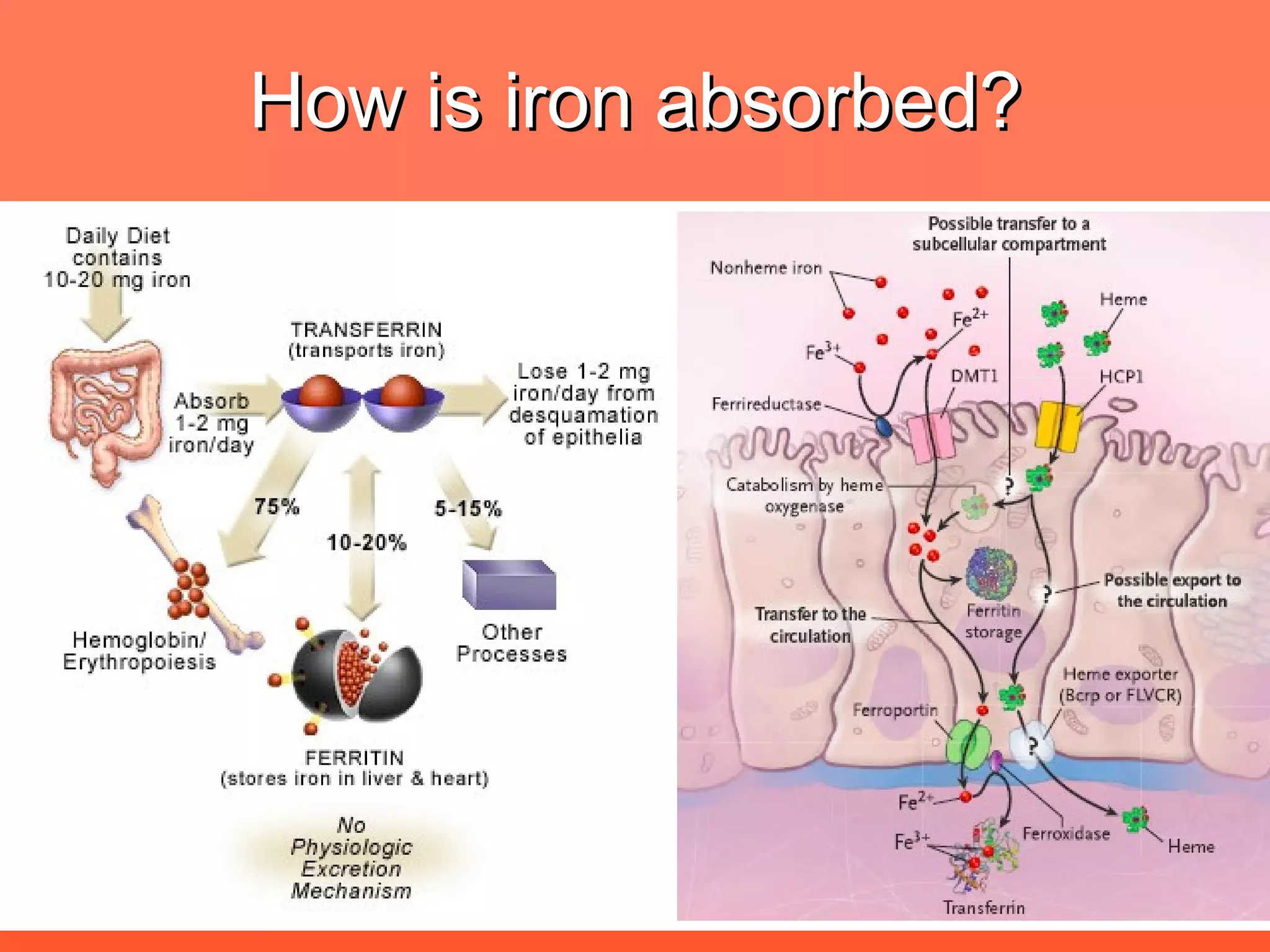
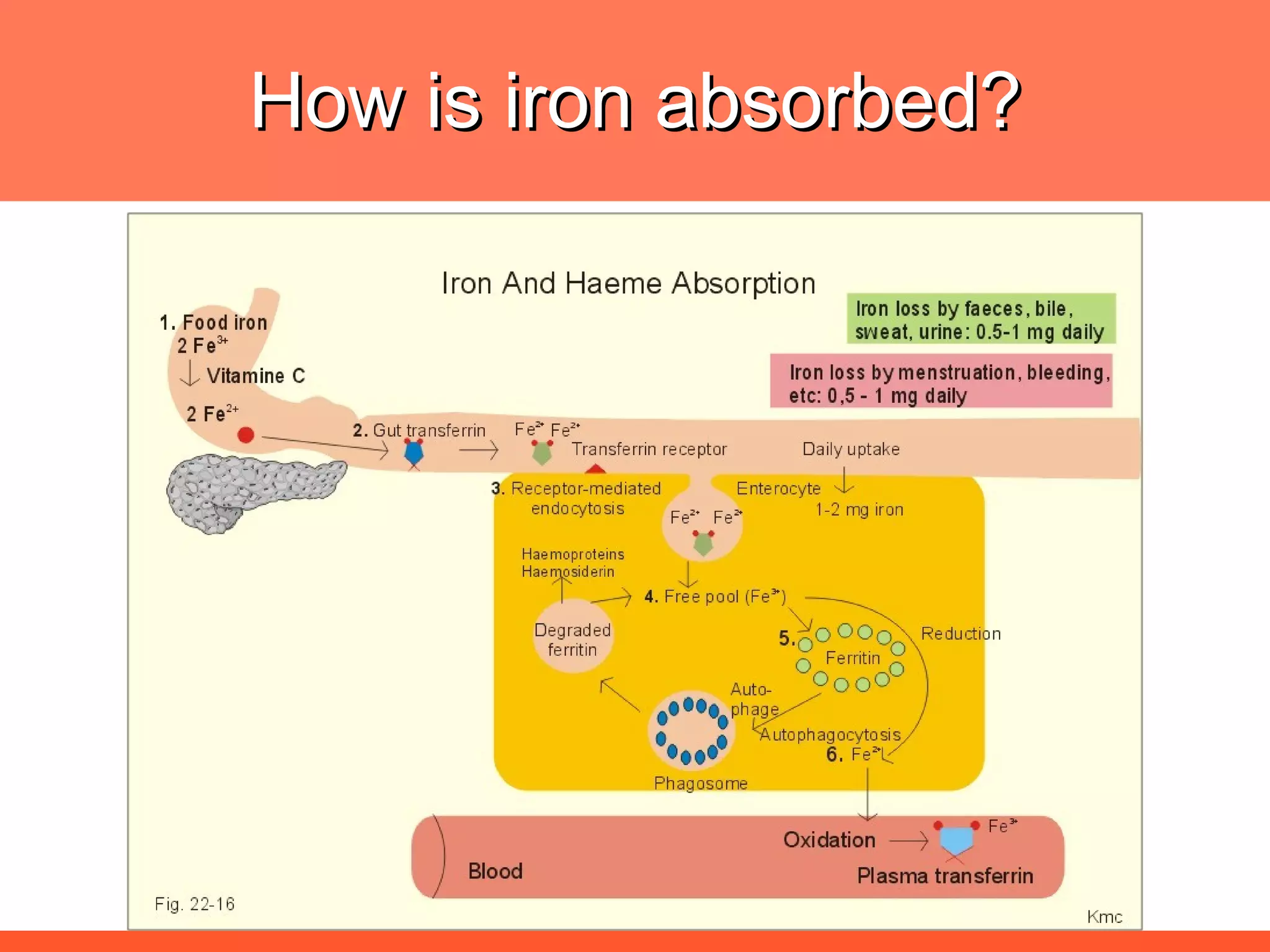
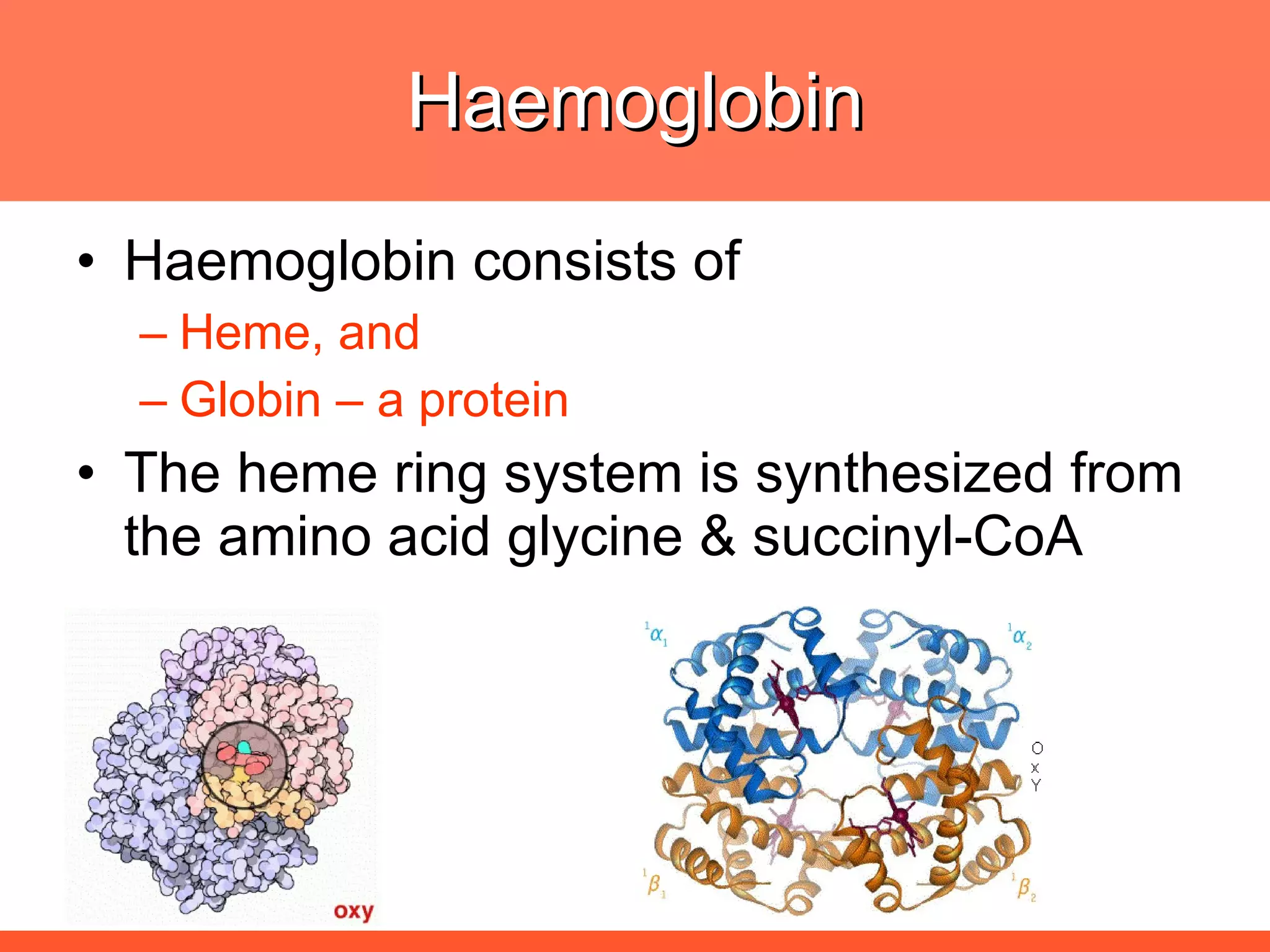
Iron deficiency anemia is caused by a lack of iron needed to produce hemoglobin. There are multiple factors that influence iron absorption, transport, storage and utilization in hemoglobin and red blood cell production. An ideal treatment addresses these factors by providing iron along with proteins, vitamins and minerals to efficiently synthesize and mature hemoglobin and red blood cells.


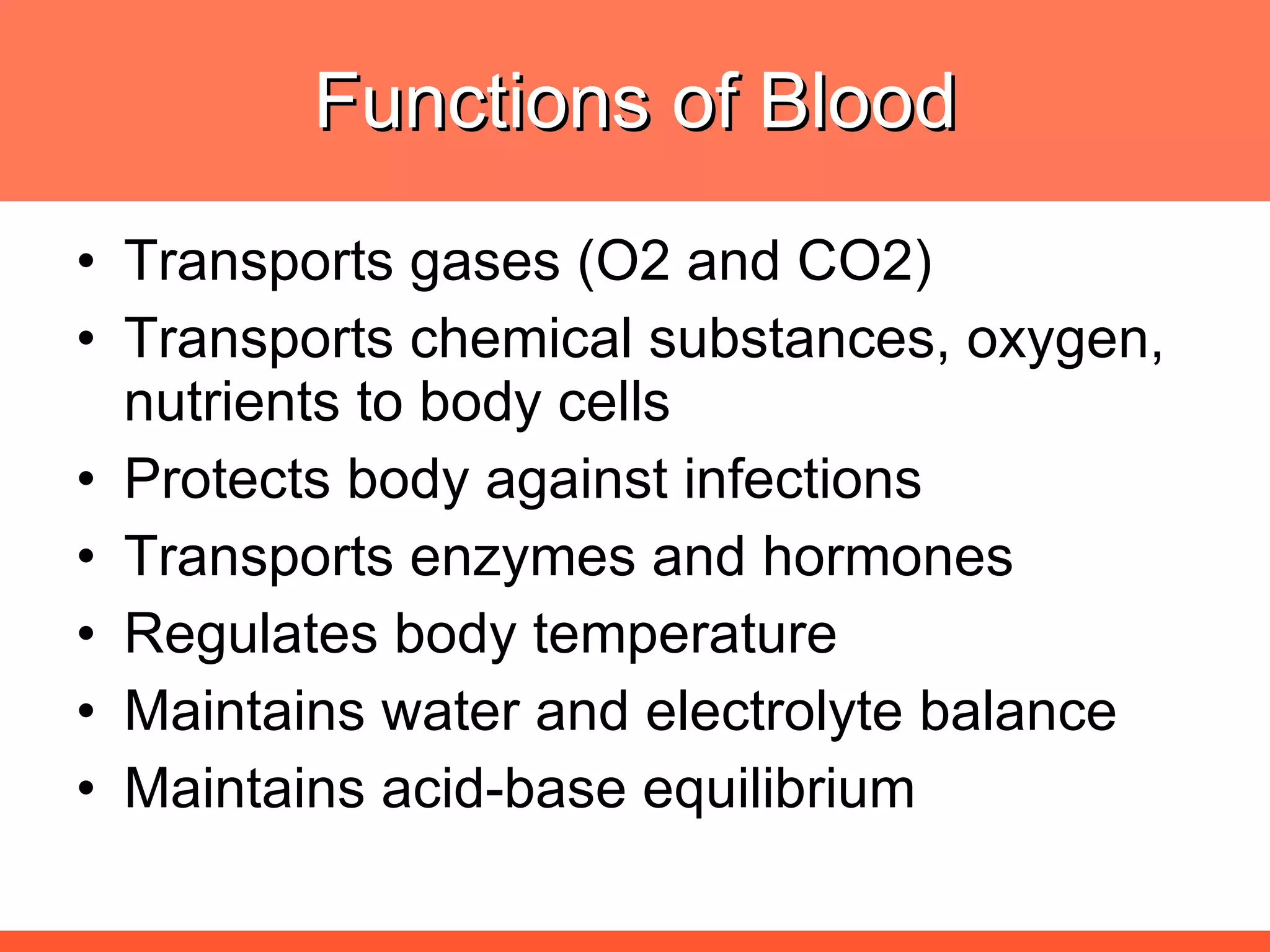
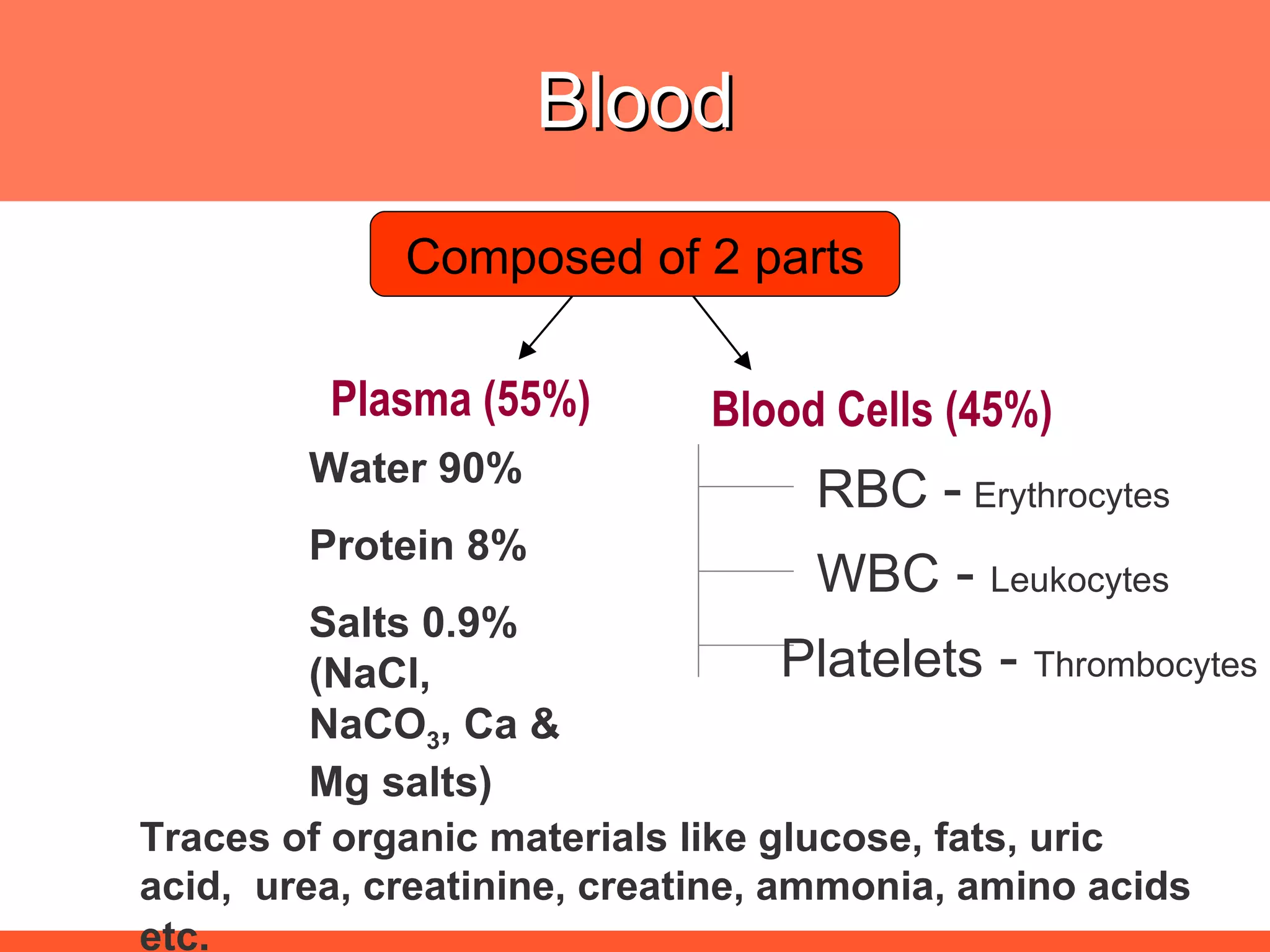
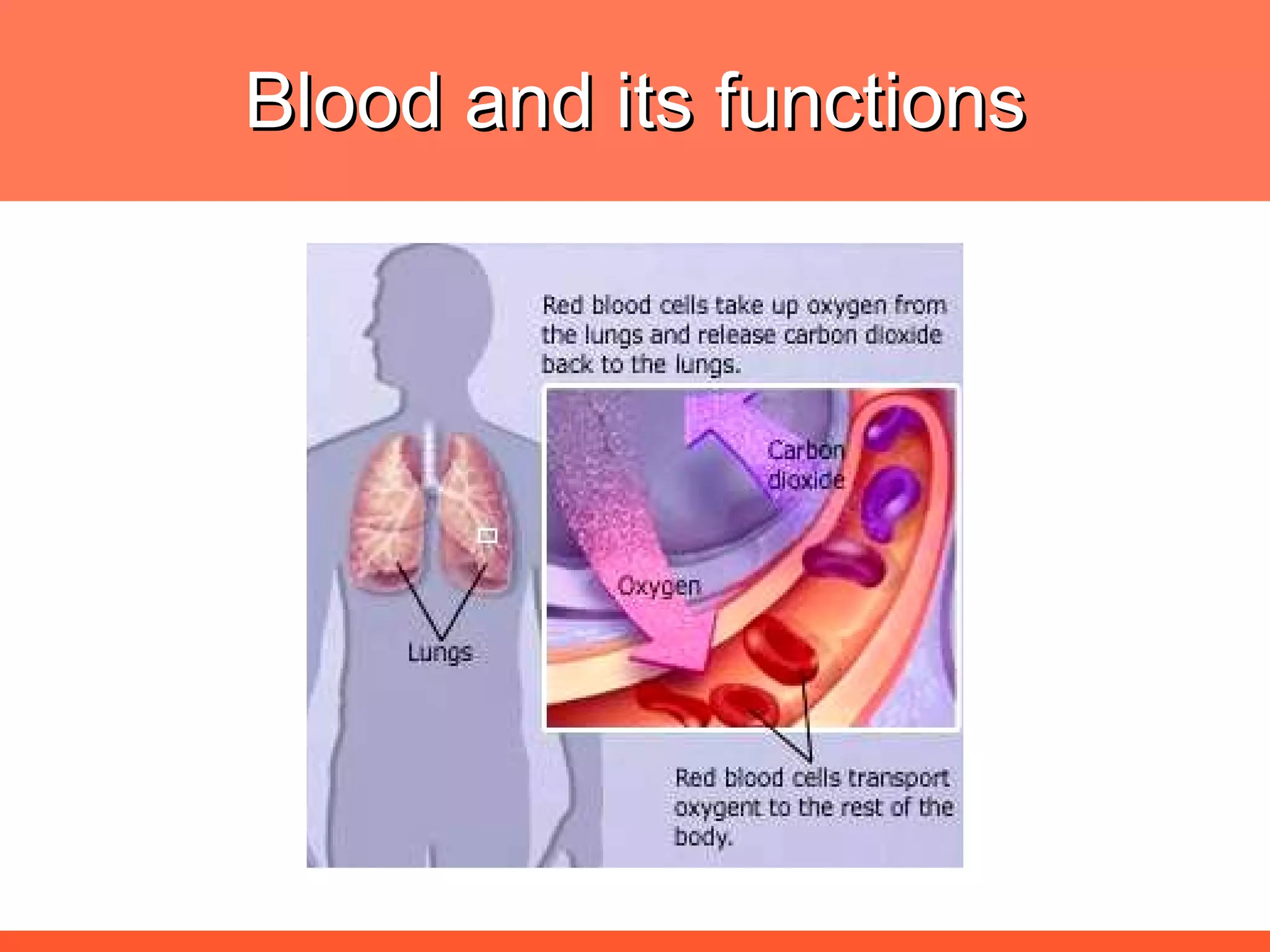













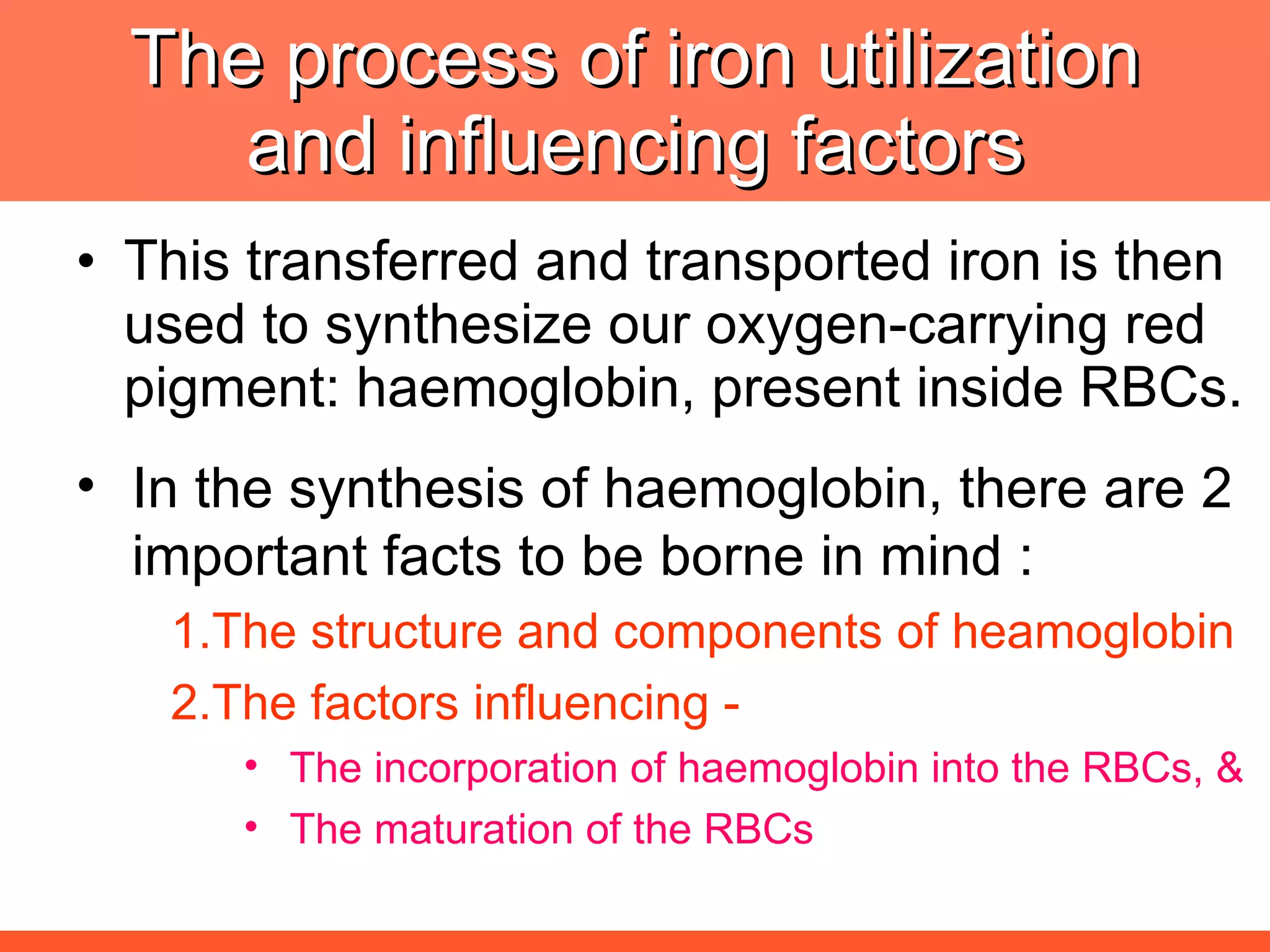
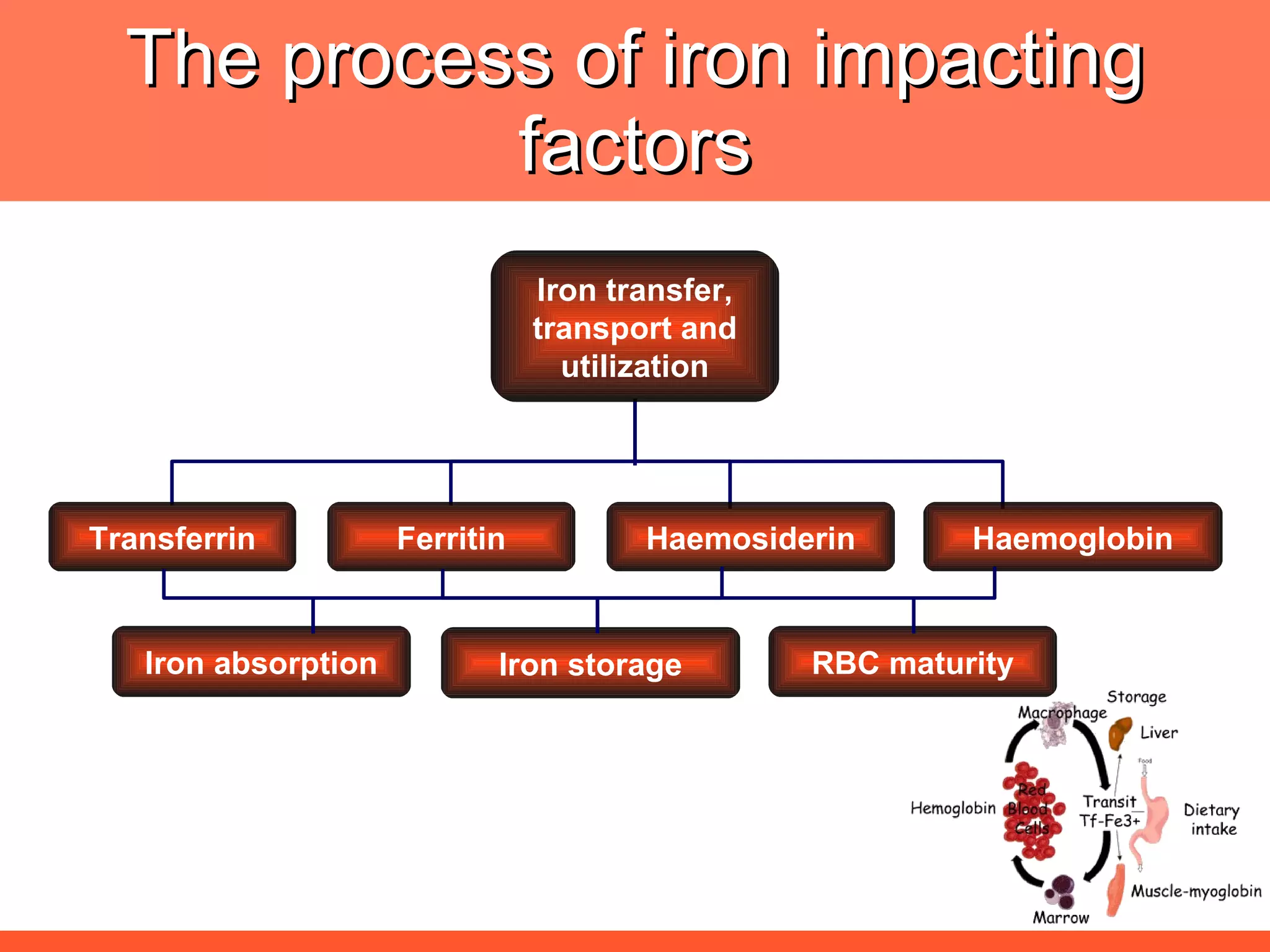
![The needs for efficient iron deficiency management Thus, an ideal approach in impacting [IDA] iron deficiency anaemia is one with multiple interventions that will affect: Efficient synthesis and functioning of the transport proteins Efficient synthesis of haemoglobin [Hb] Efficient maturation of the RBCs Efficient Hb synthesis demands in addition to iron; factors that affect iron transport/storage, Hb synthesis & RBC maturity process](https://image.slidesharecdn.com/anemiatechslides-110603221643-phpapp02/75/Anemia-tech-slides-43-2048.jpg)

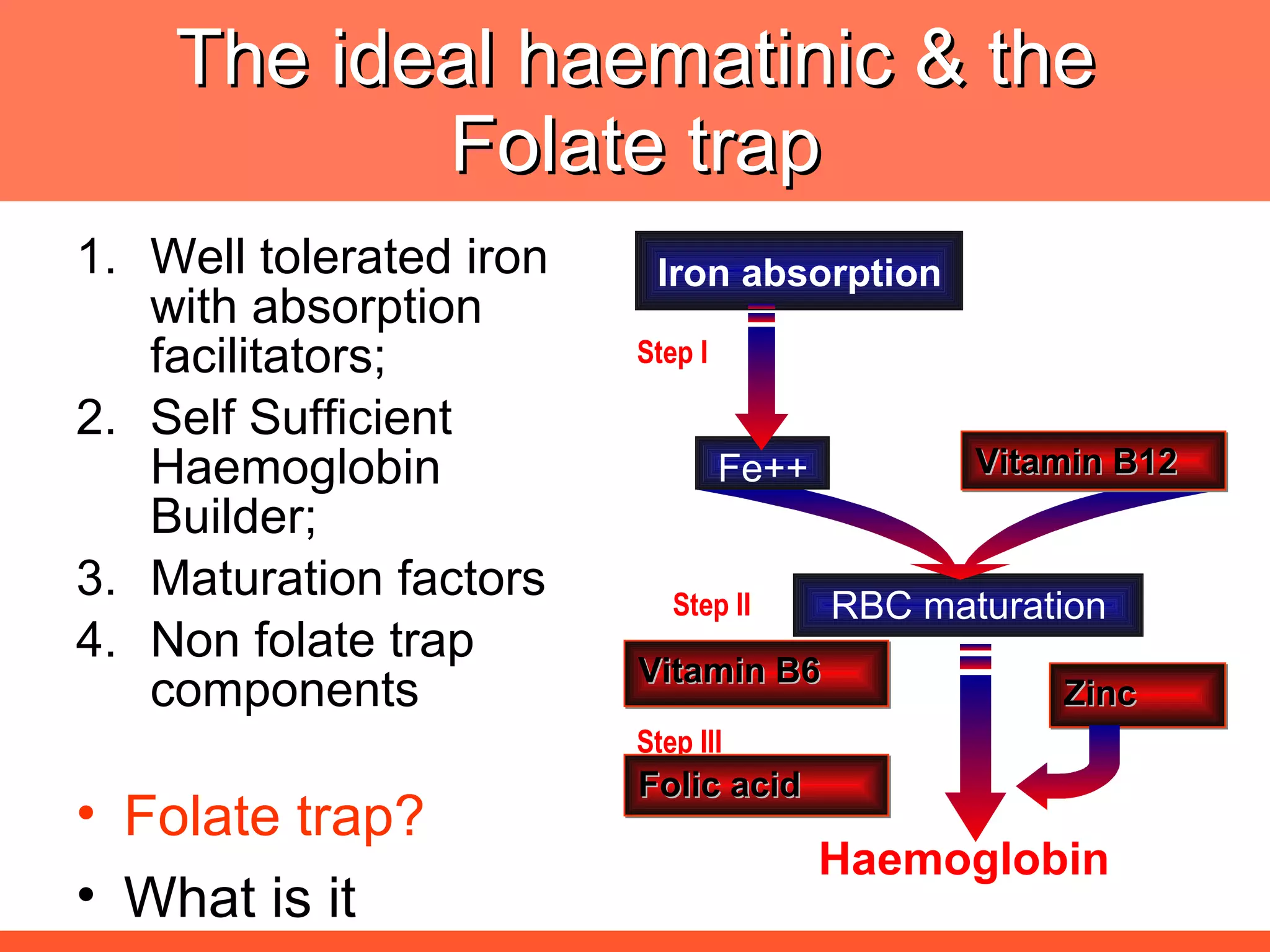
![The ideal haematinic Well tolerated iron with absorption facilitators [Vit, C] and performance enhancers [protein]; Maturation factors [Vit B6, Vit. B12, Folic acid] Non folate trap components Self Sufficient Haemoglobin Builder; Folate trap? What is it](https://image.slidesharecdn.com/anemiatechslides-110603221643-phpapp02/75/Anemia-tech-slides-52-2048.jpg)