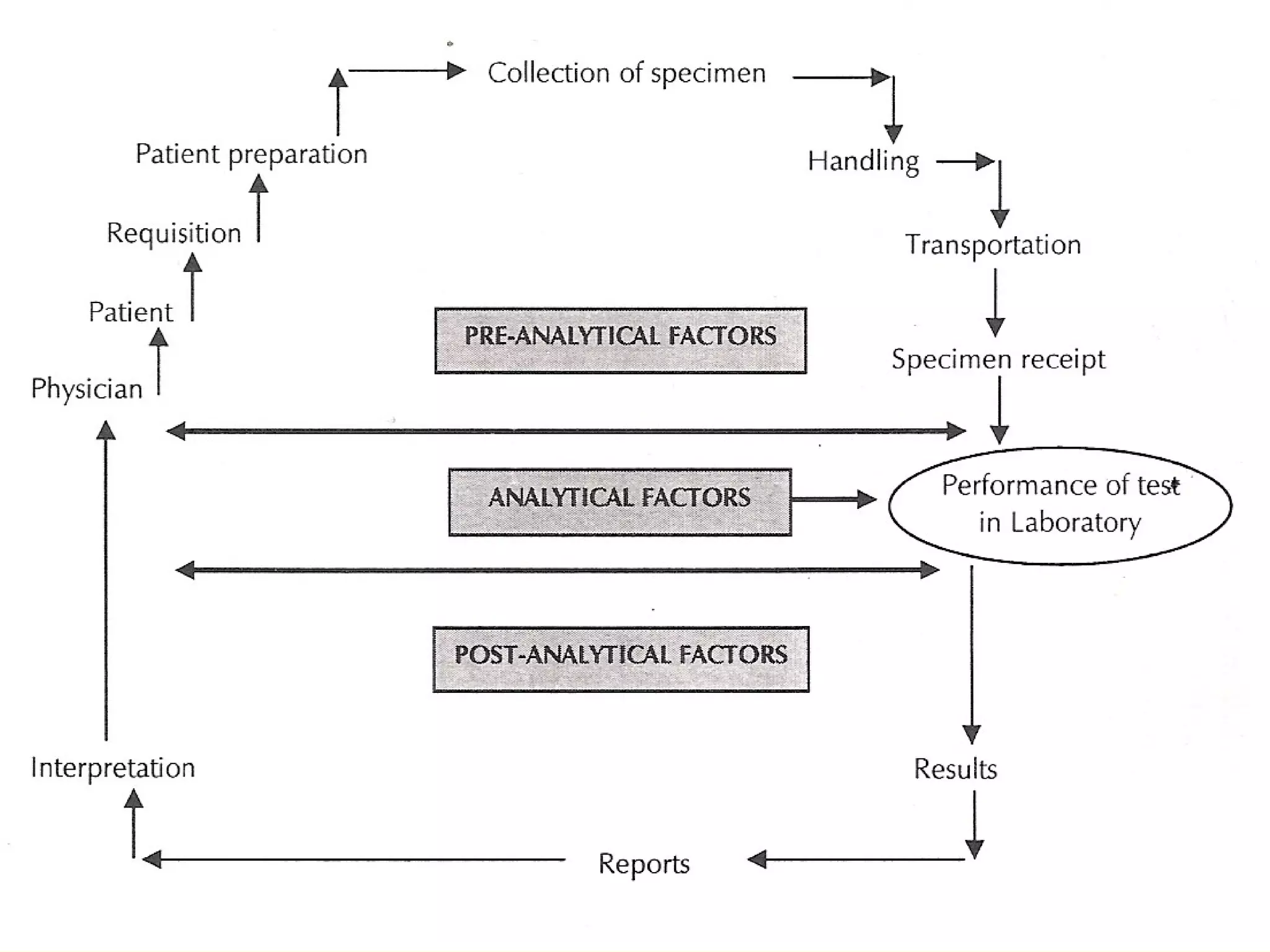
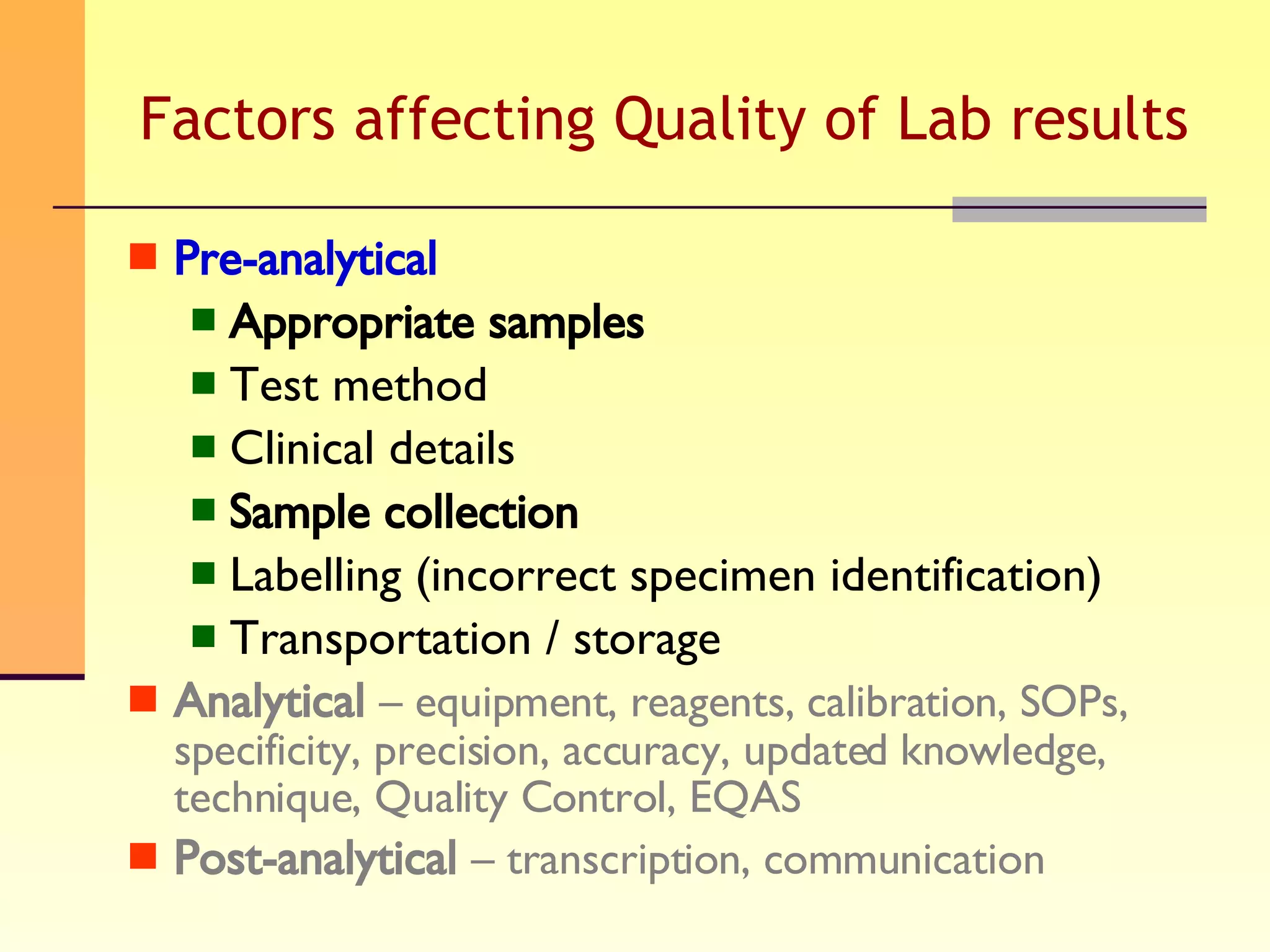
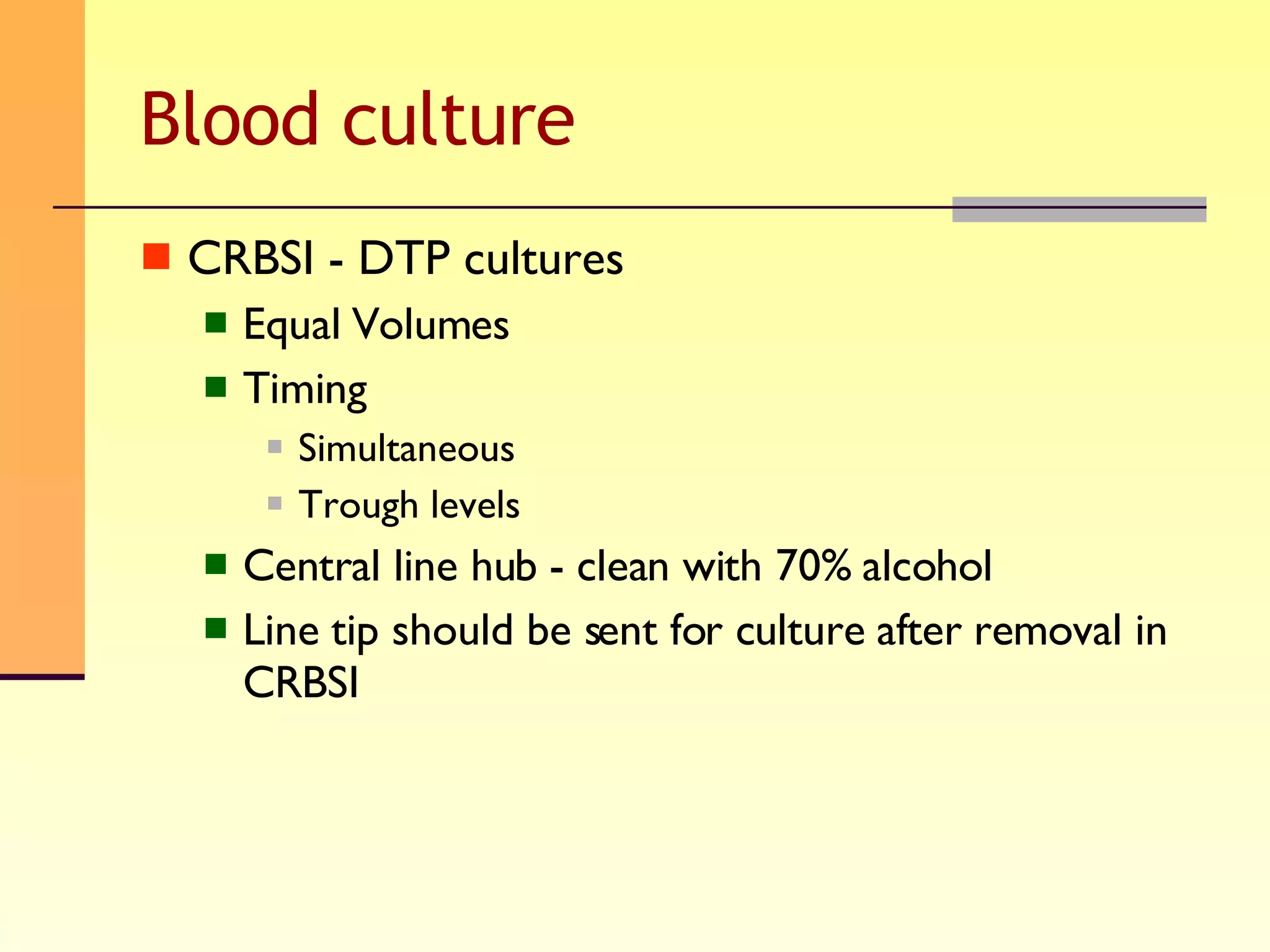
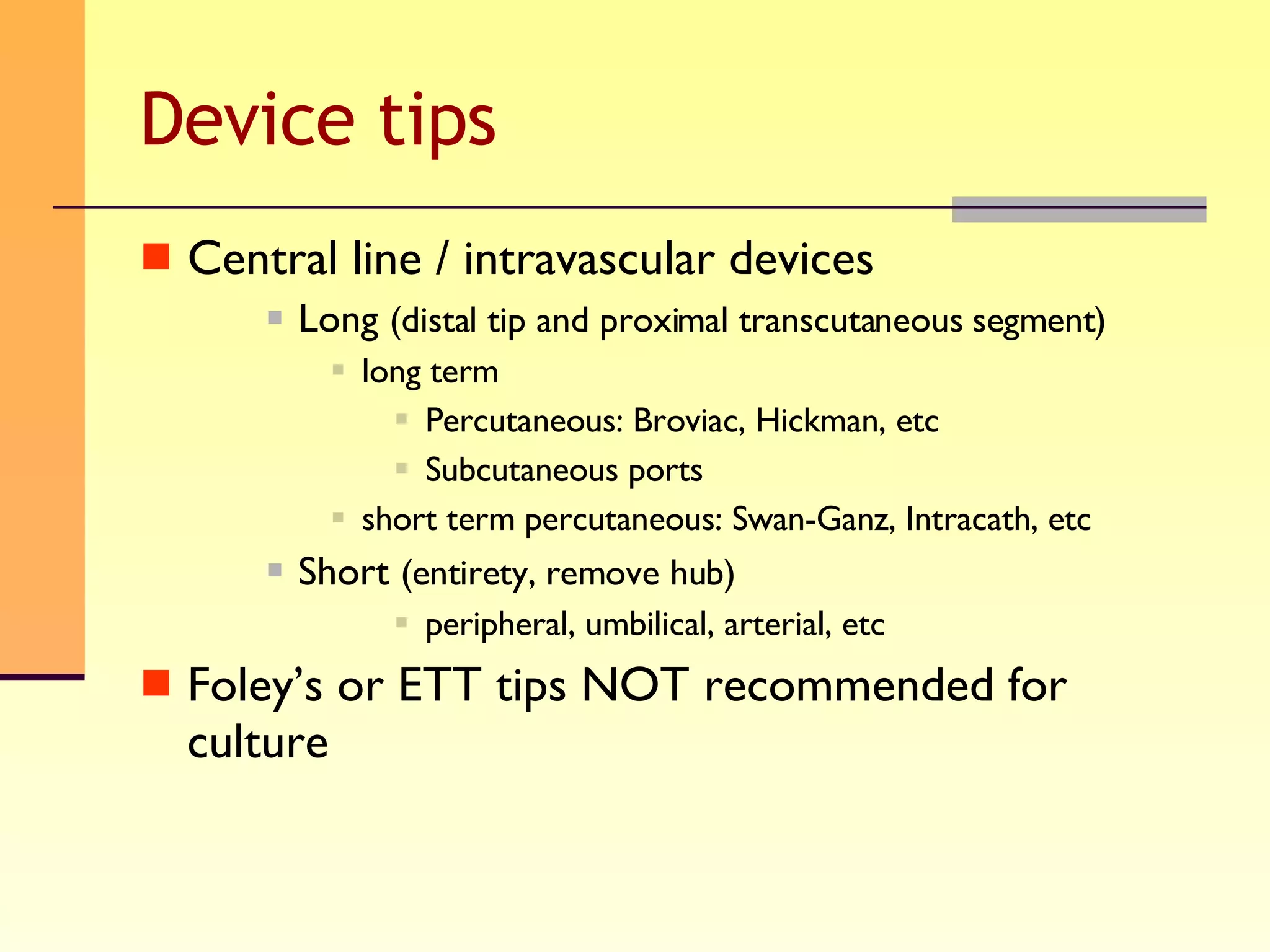
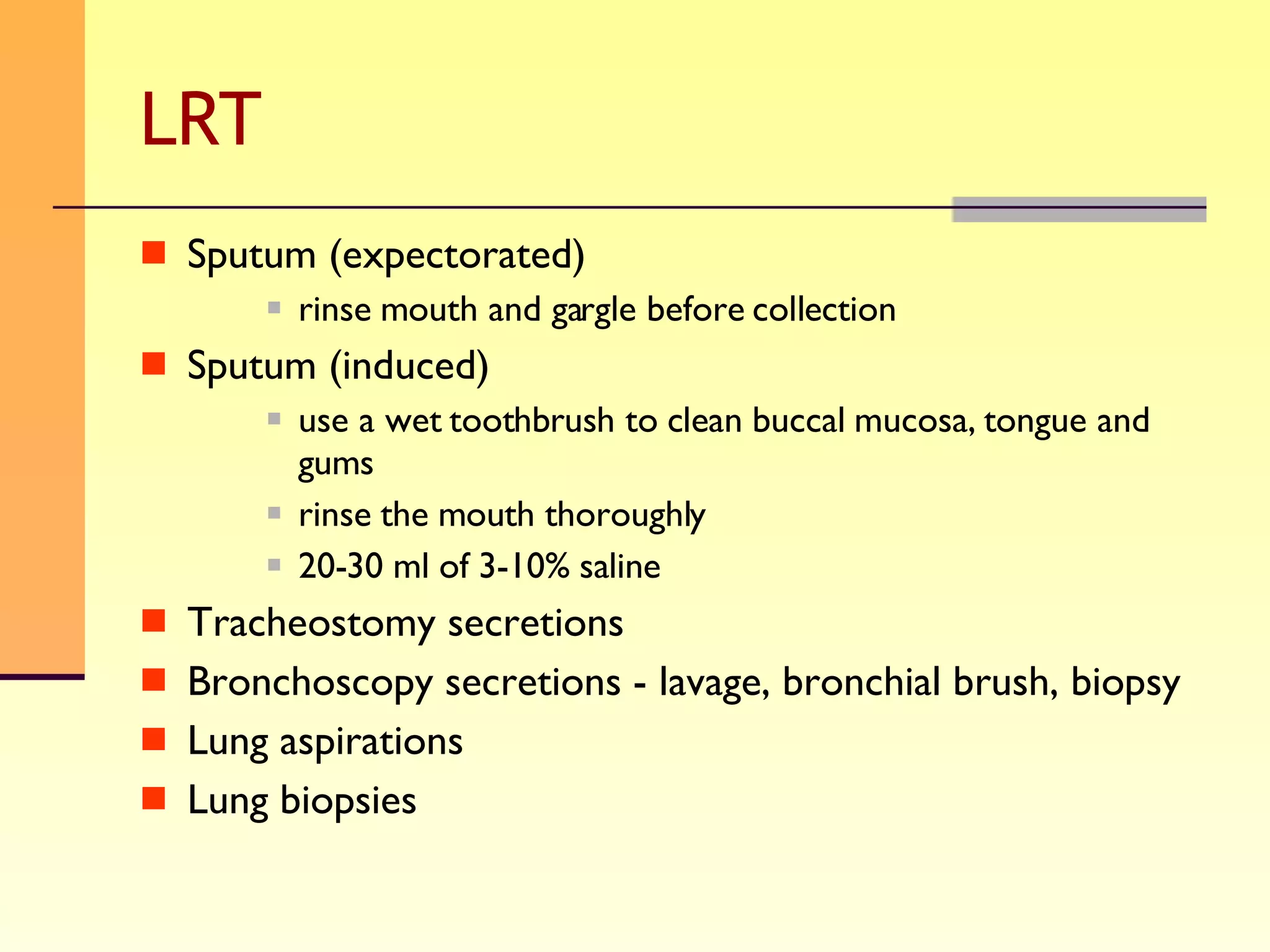
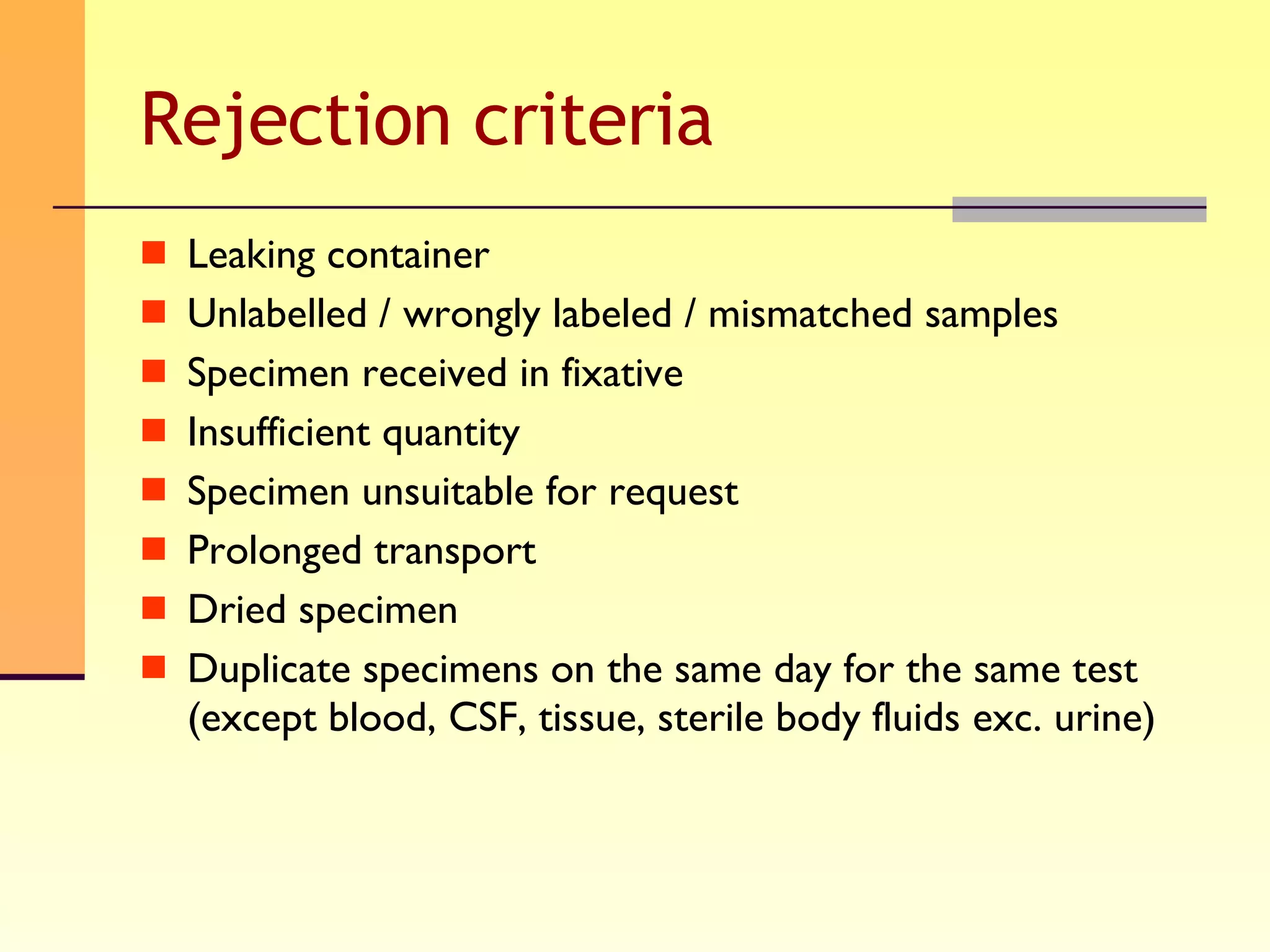
The document discusses good practices for collecting microbiology samples to obtain accurate diagnostic results. It emphasizes that sample quality depends on proper collection, transport, and clinical information provided. Specific guidelines are given for collecting various sample types like blood, respiratory, body fluids, wounds, and urine. Factors like aseptic technique, appropriate volume, and timely transportation are important for maximizing recovery of microorganisms.