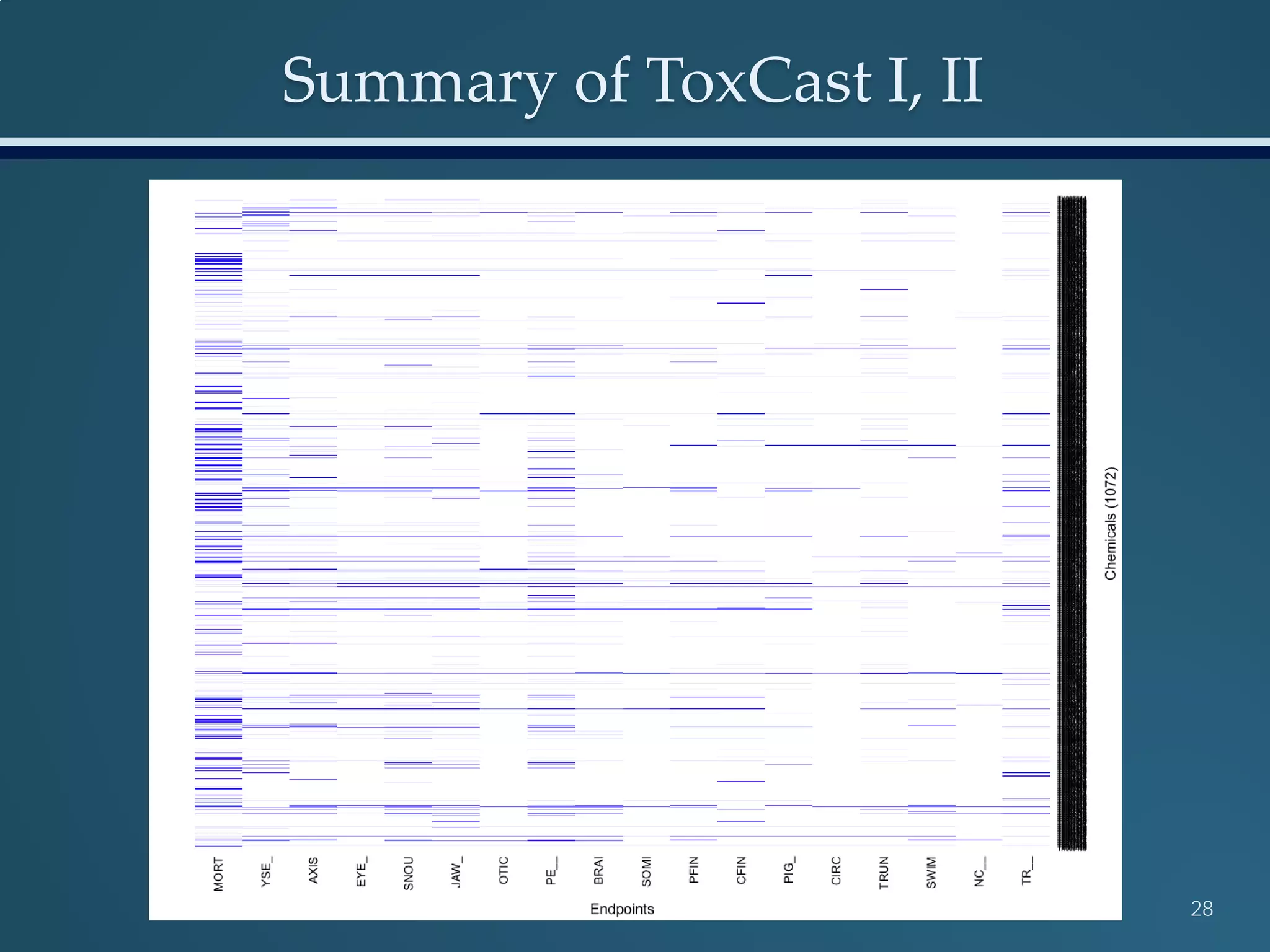
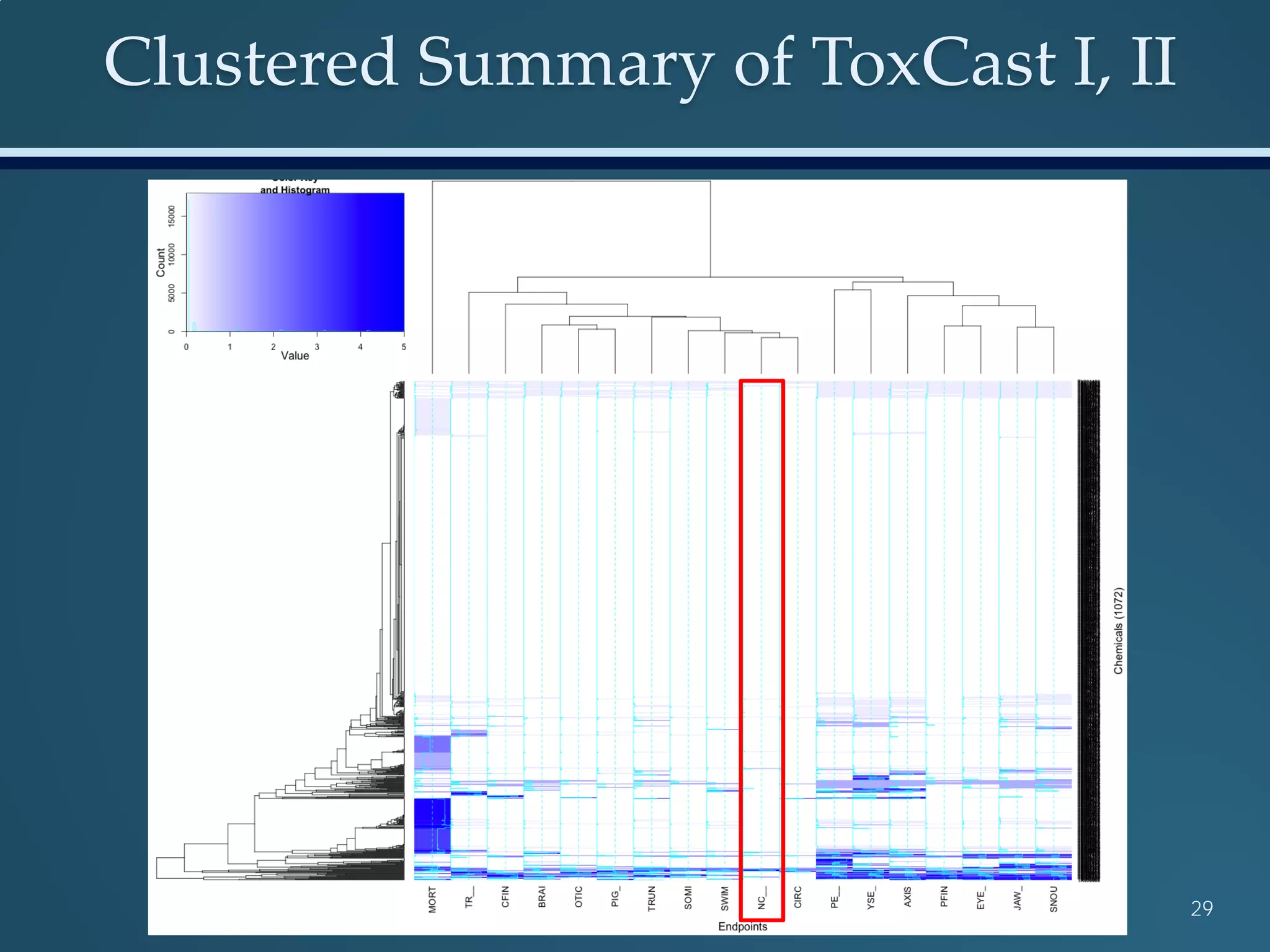
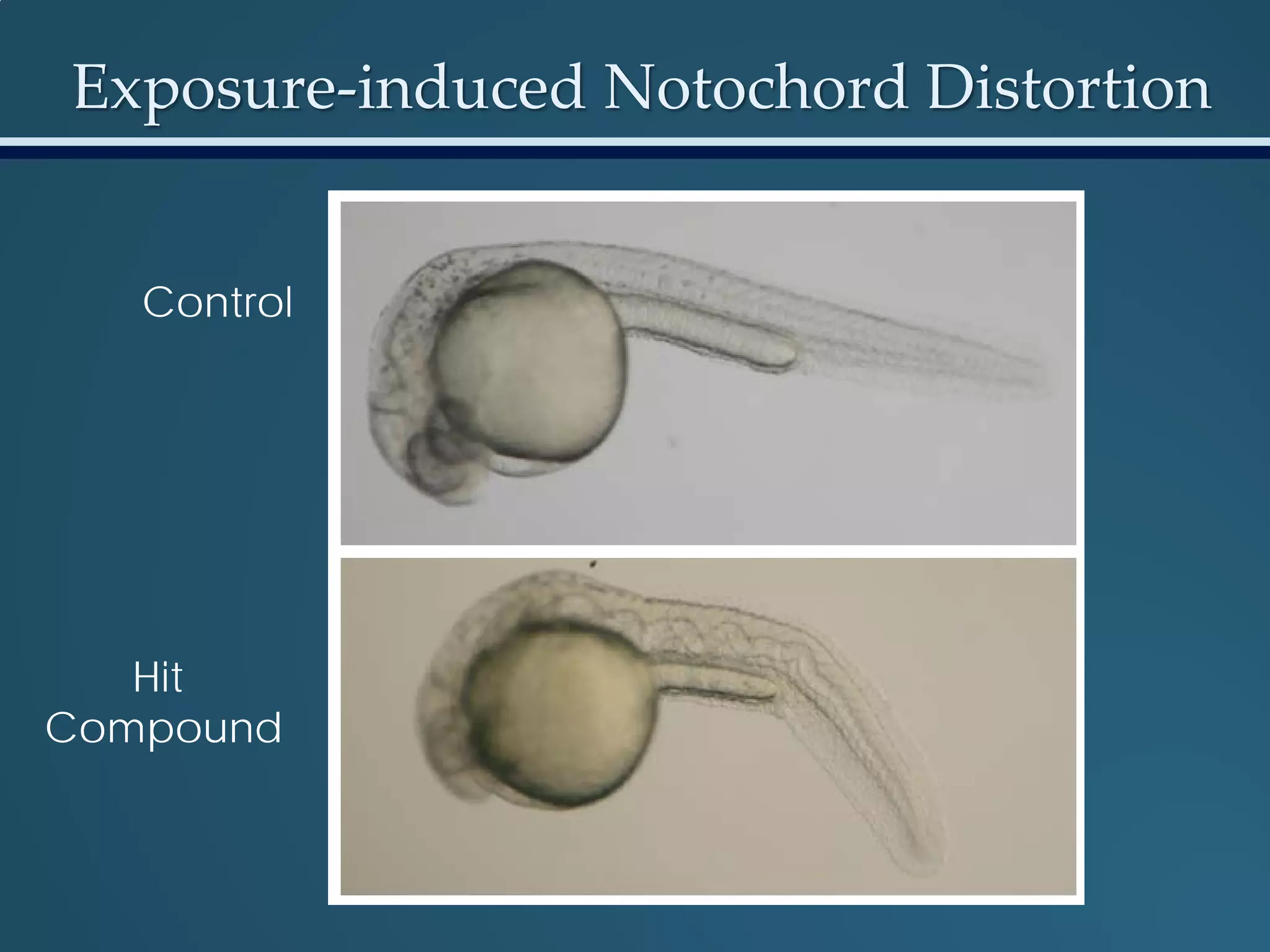
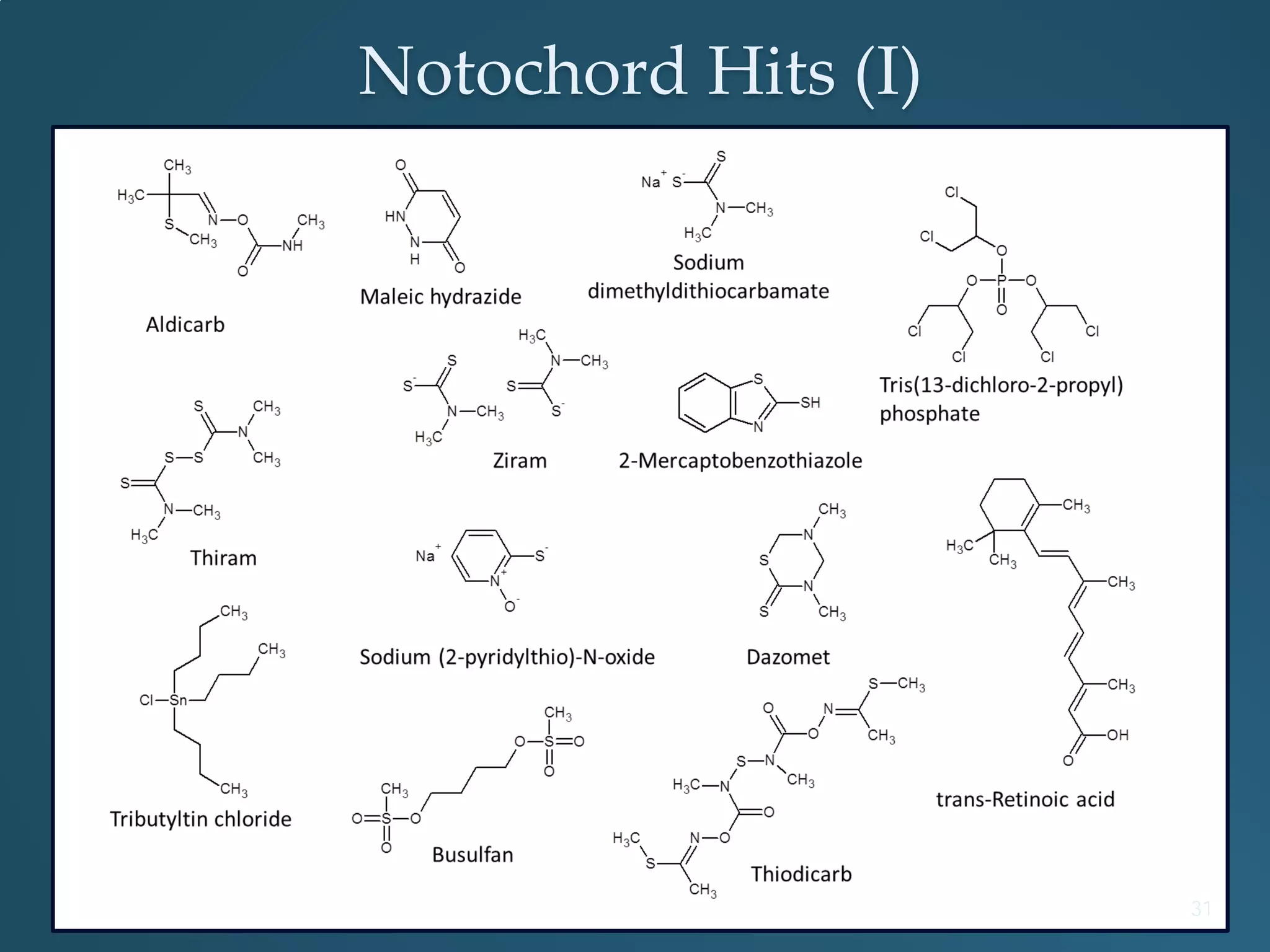
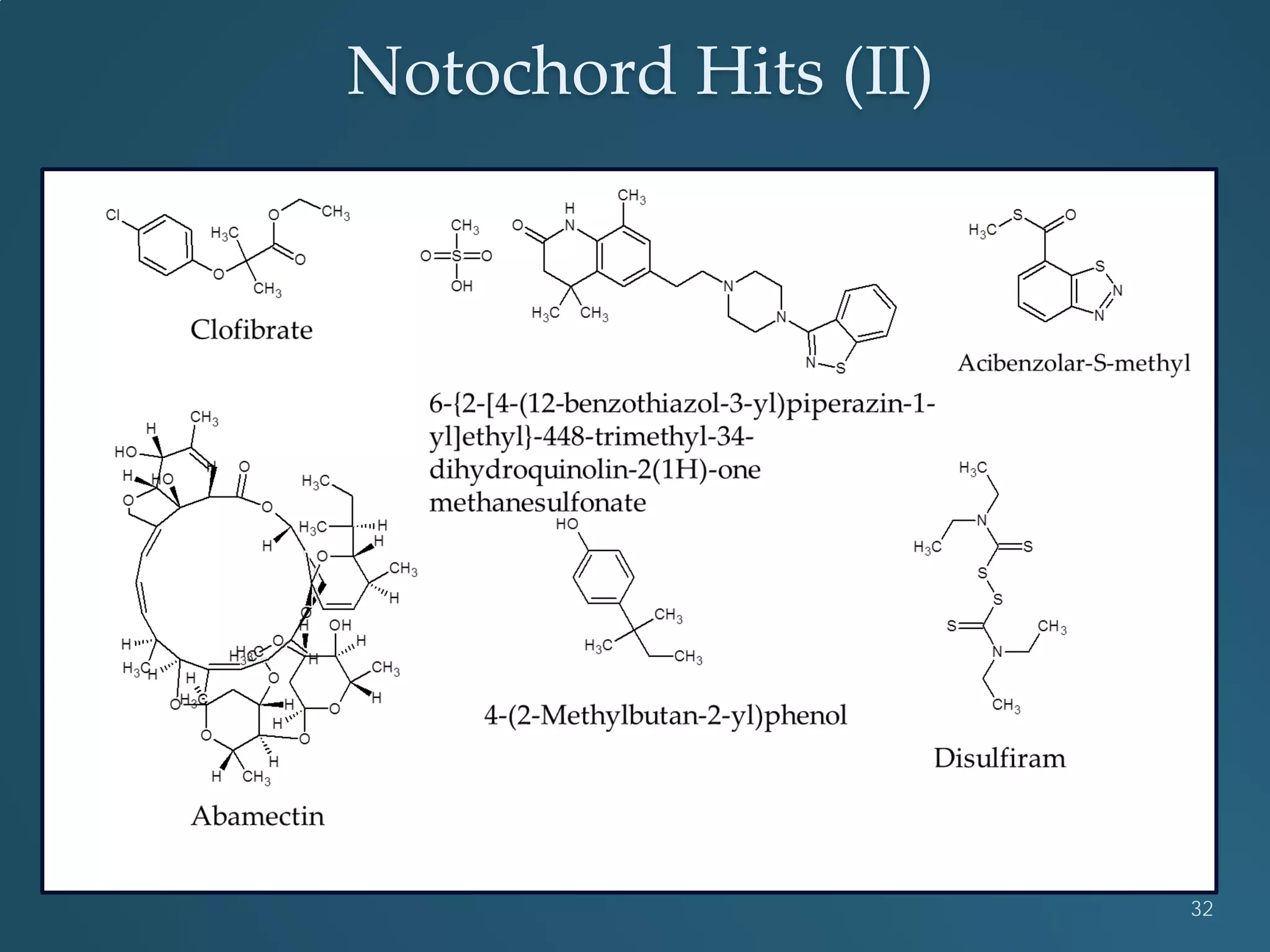
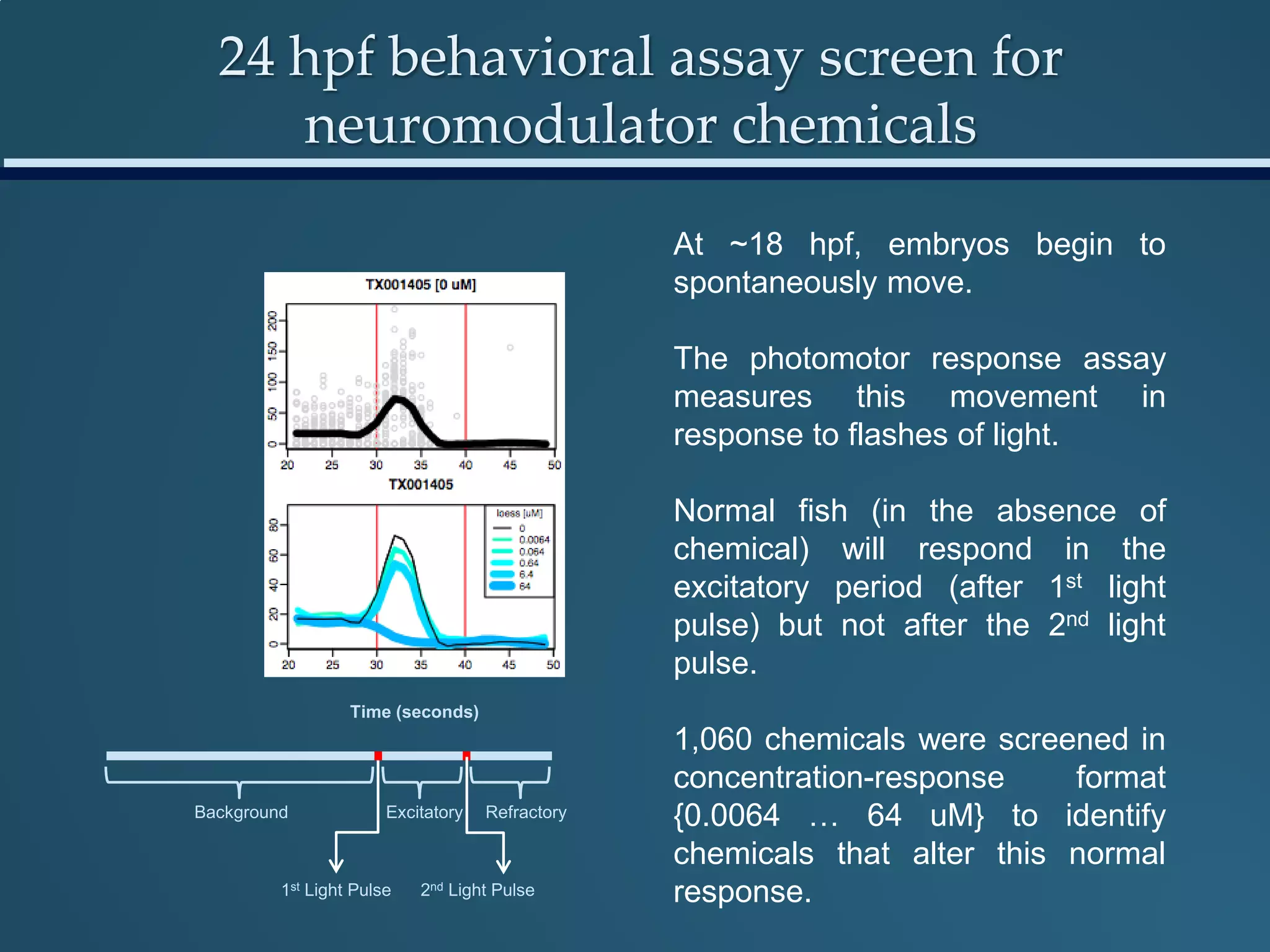
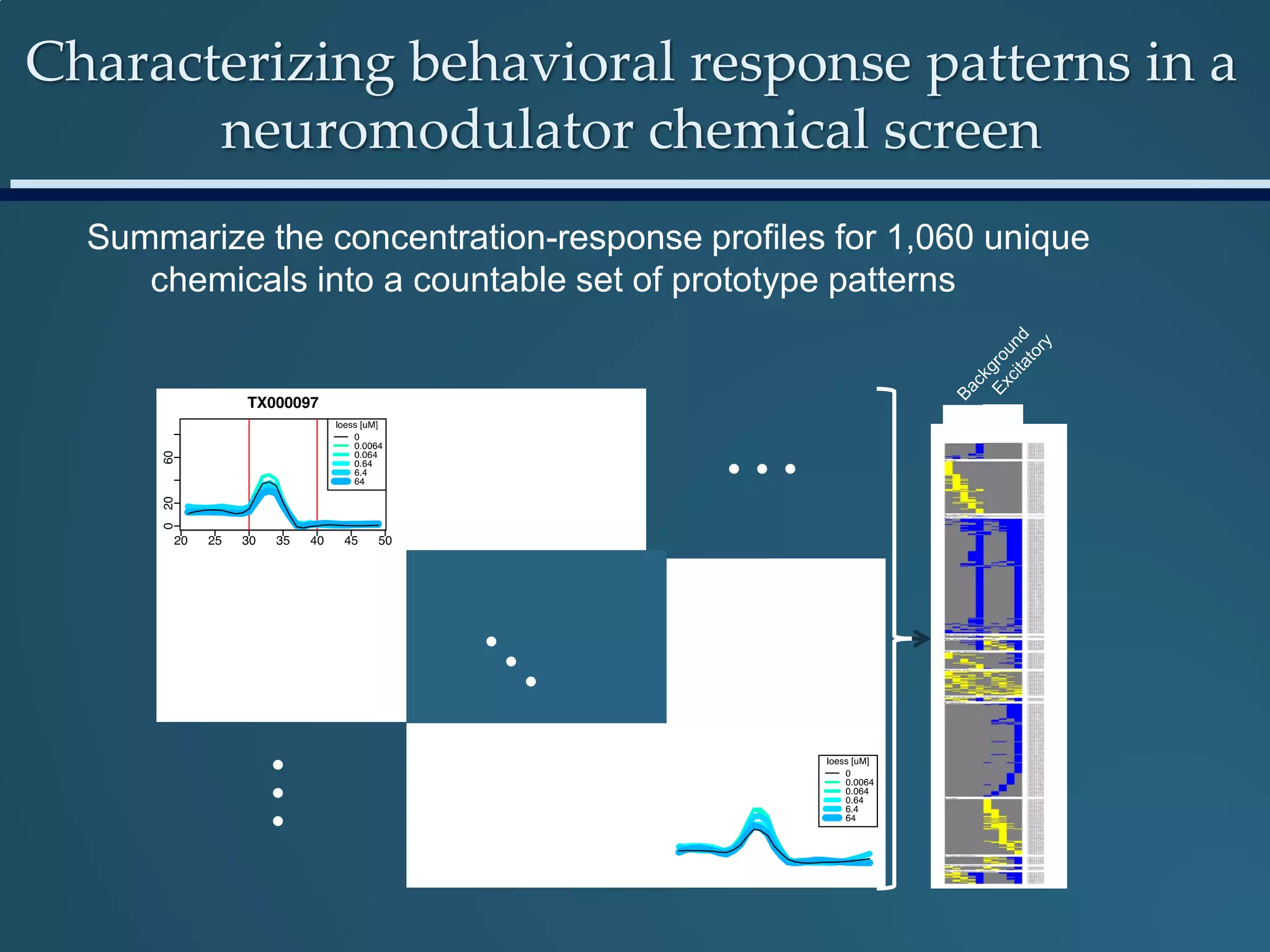
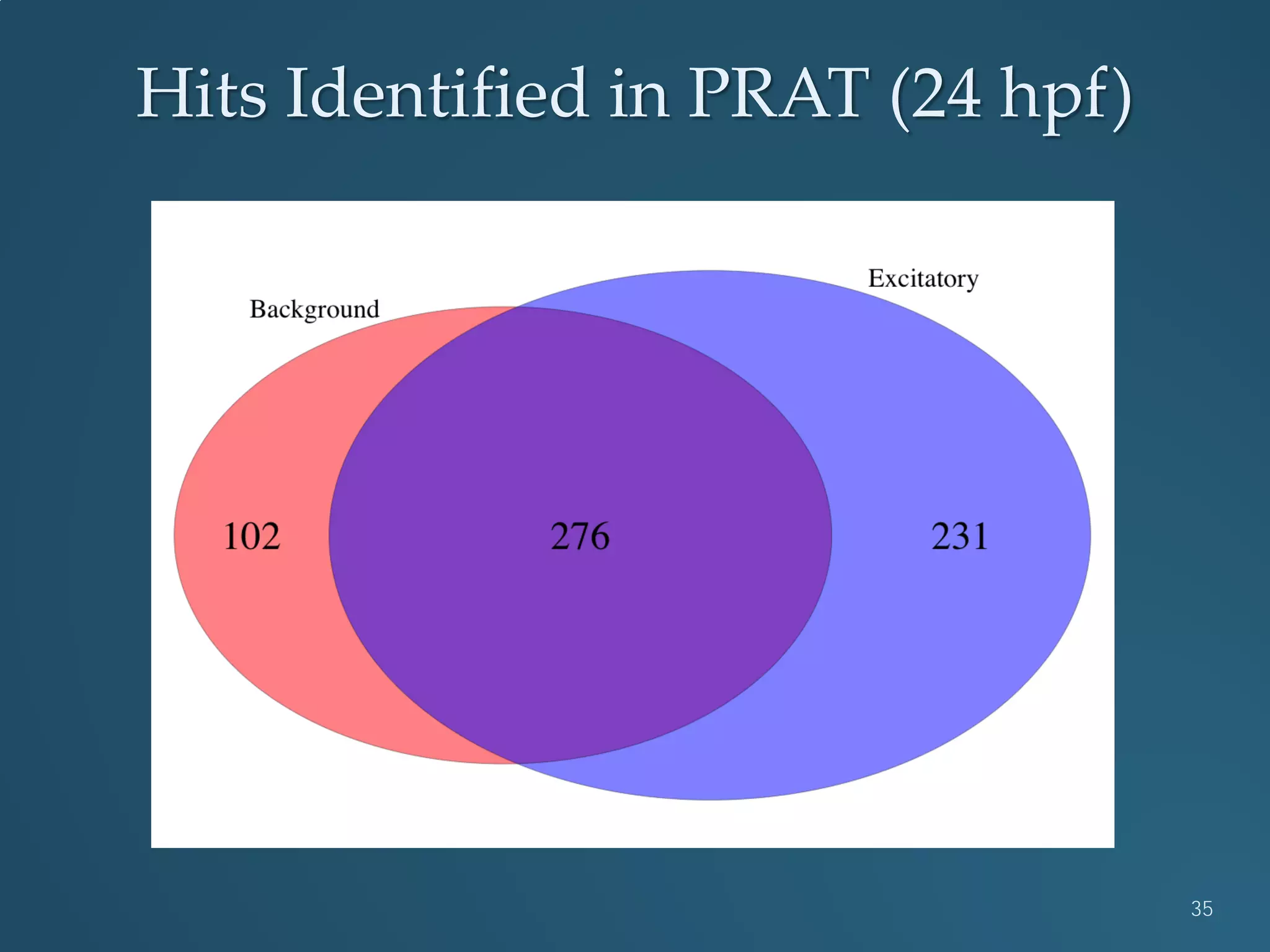
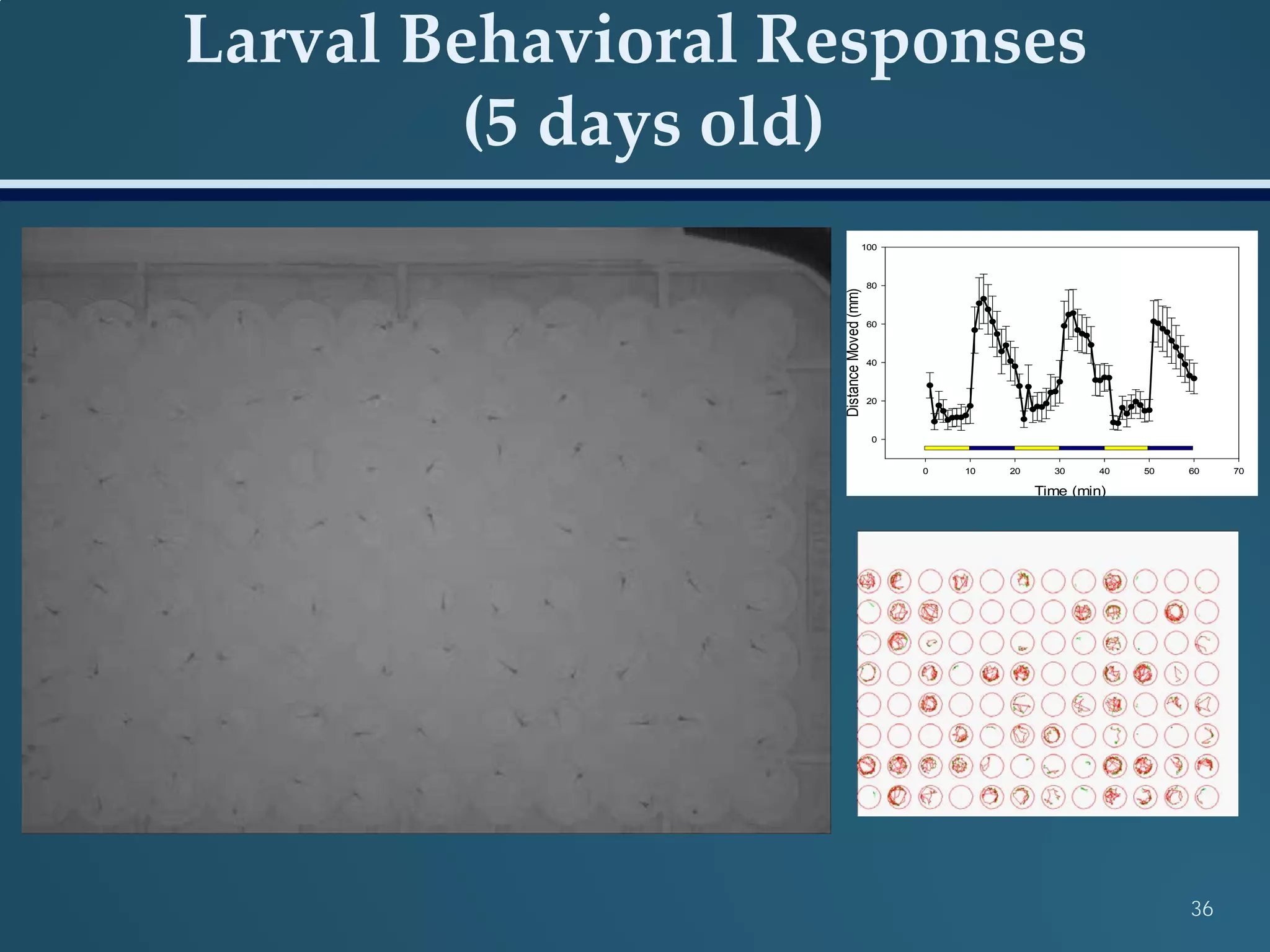
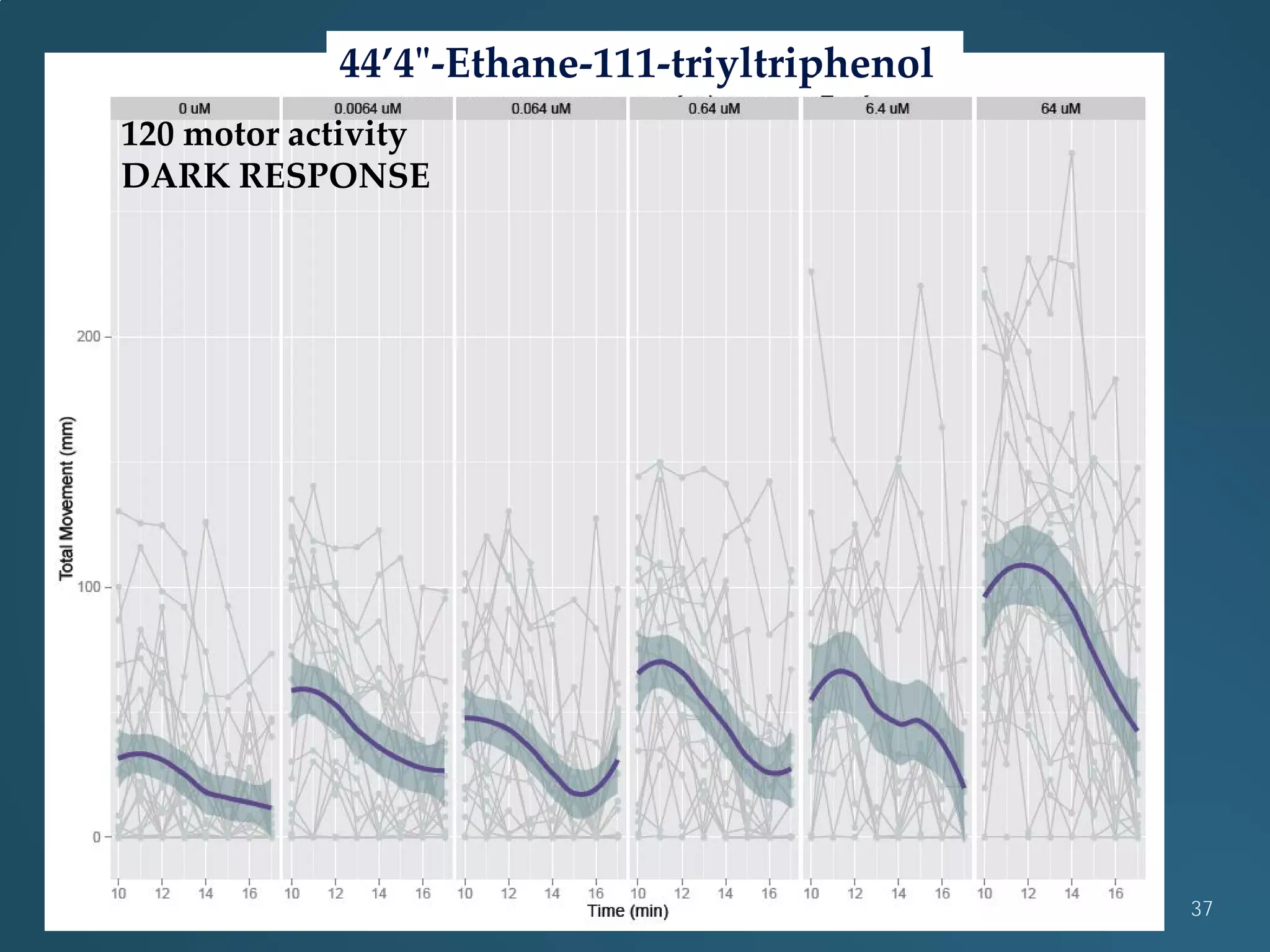
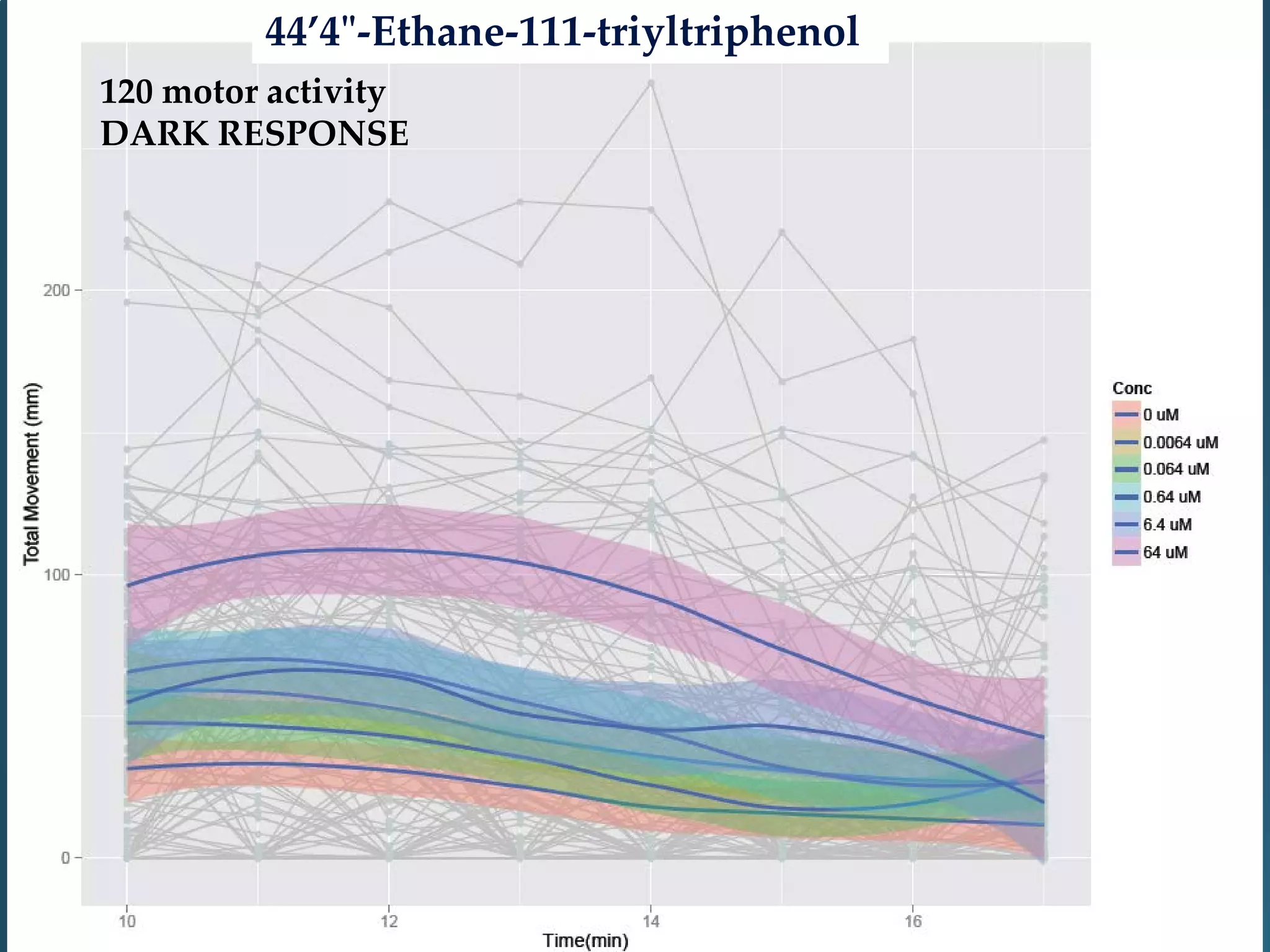
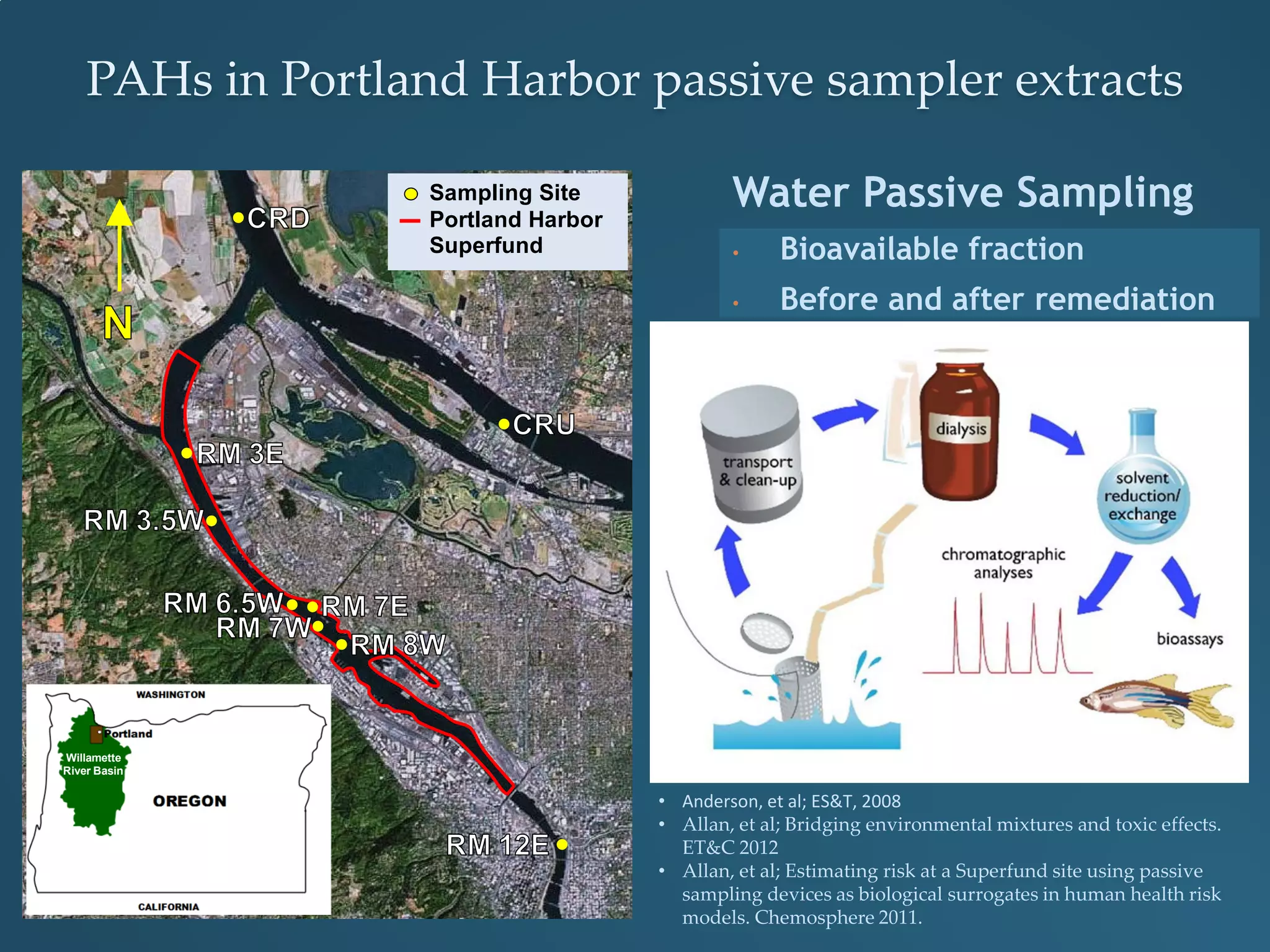
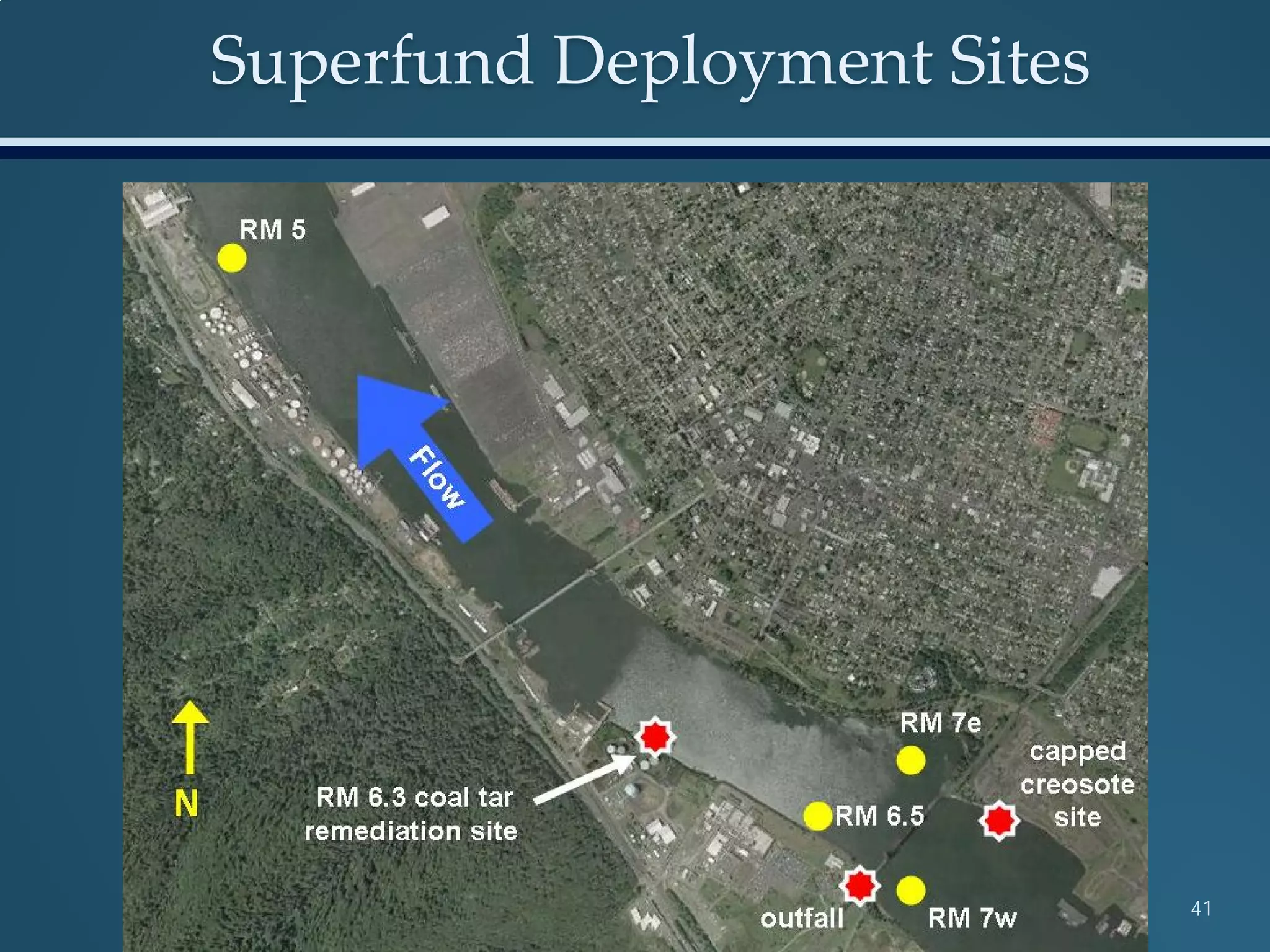
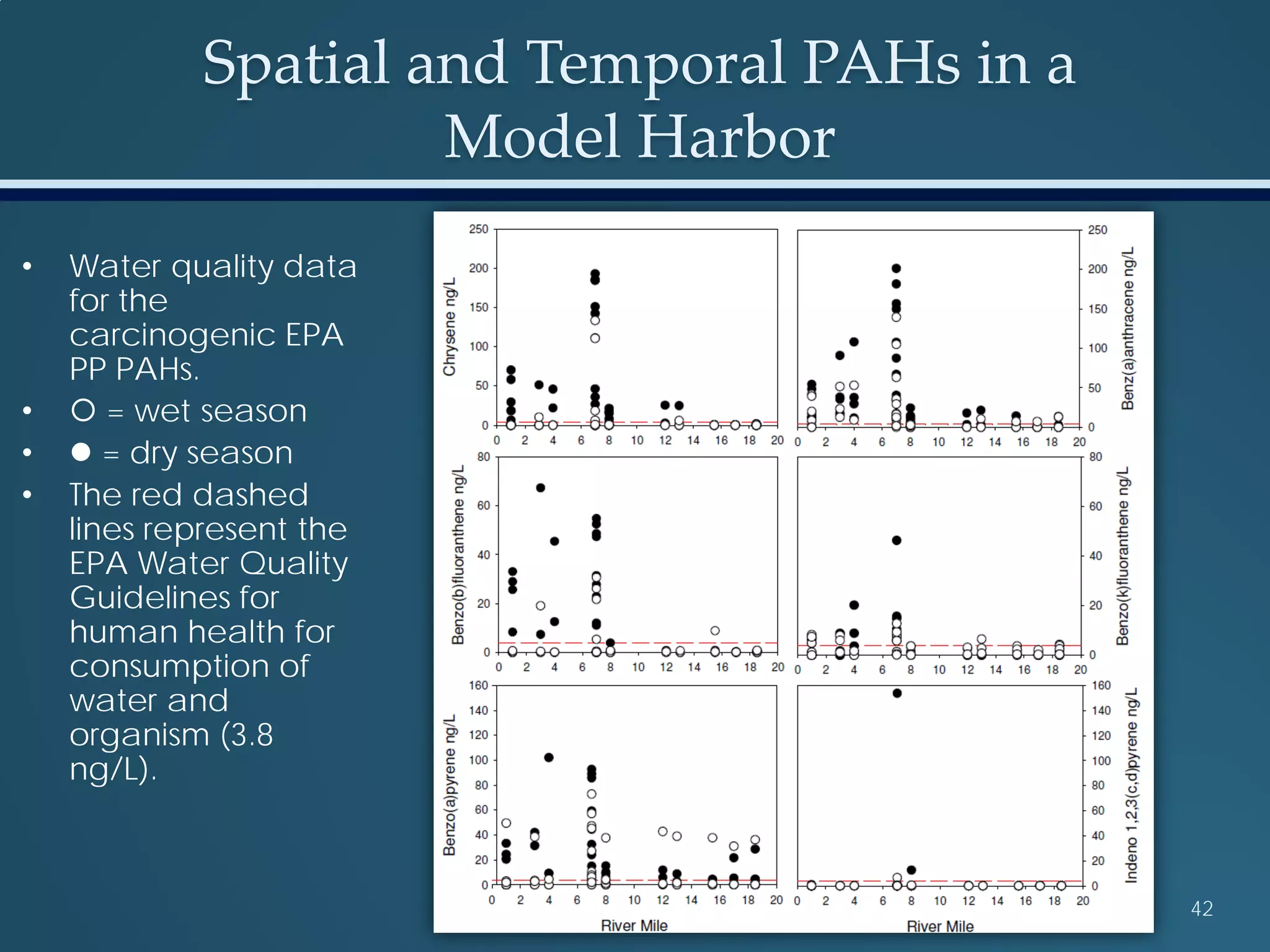
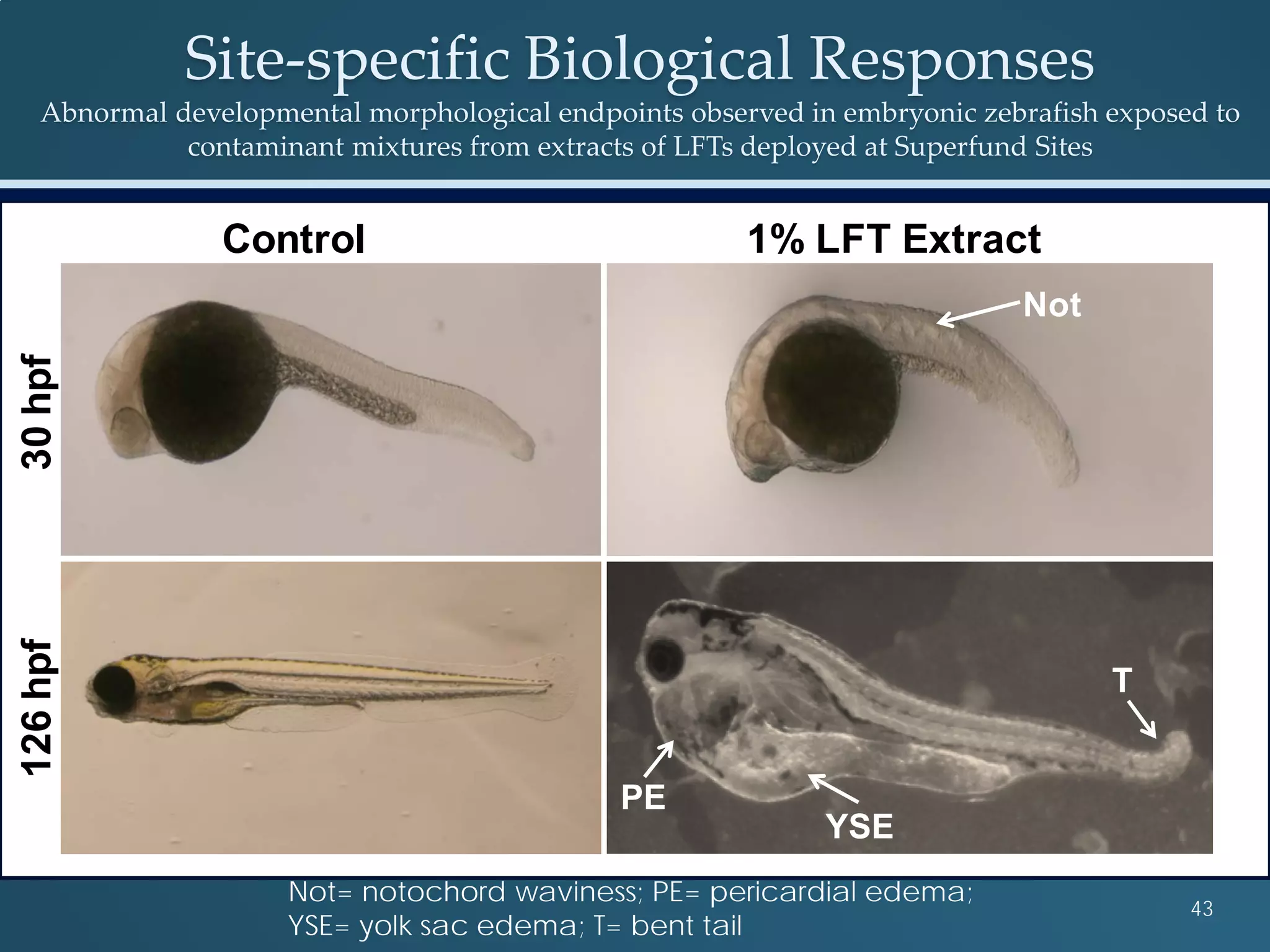
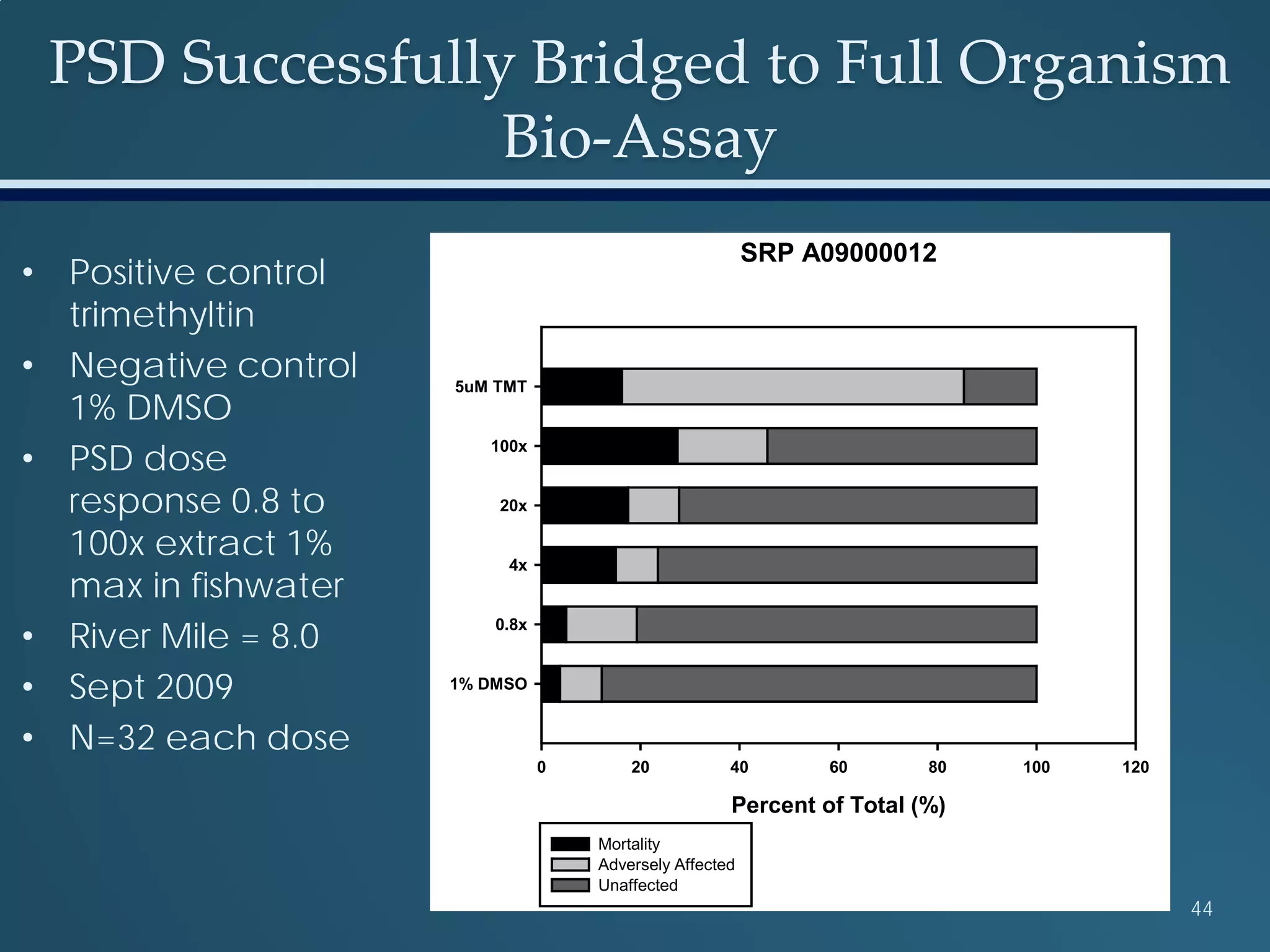
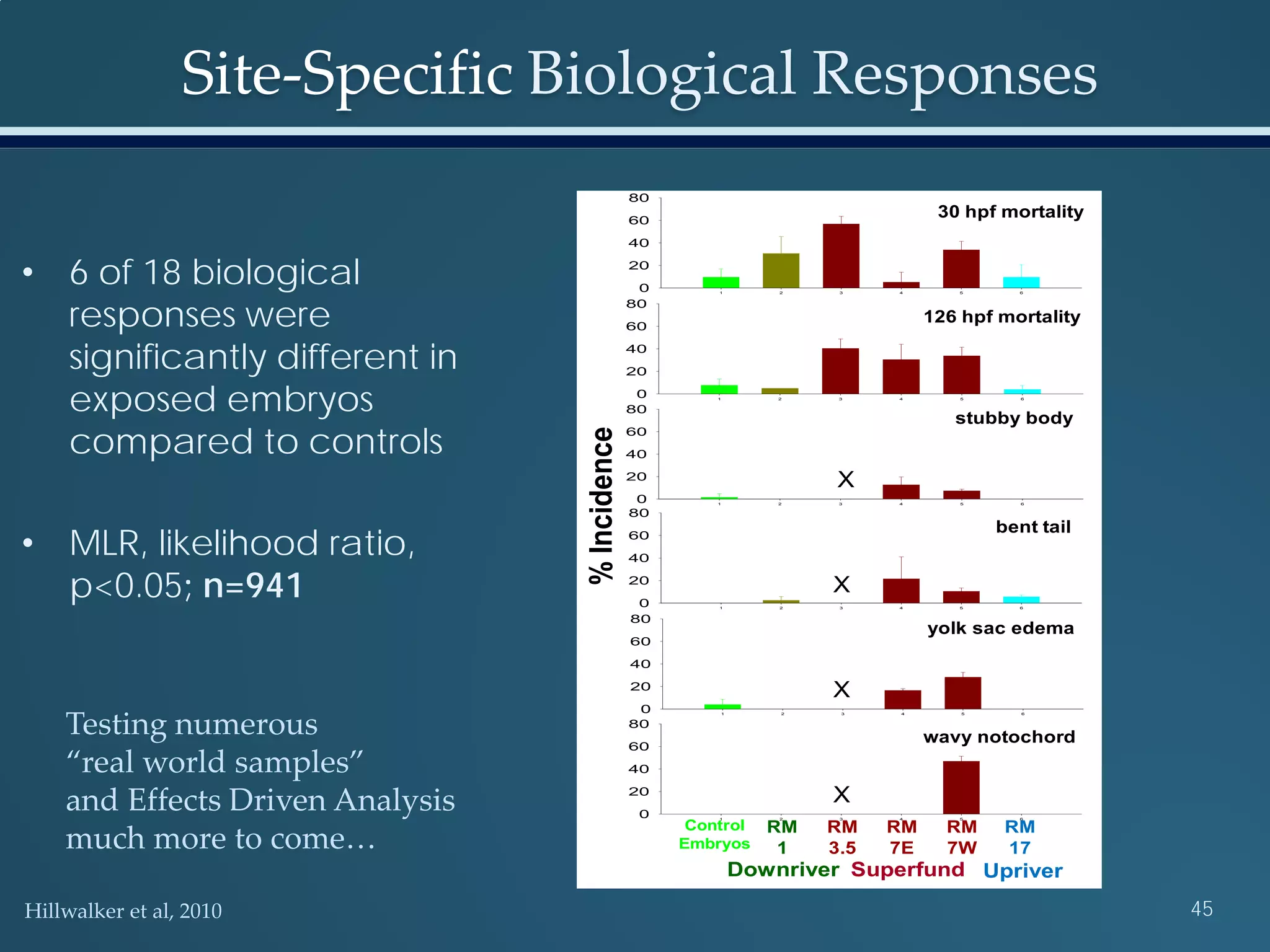
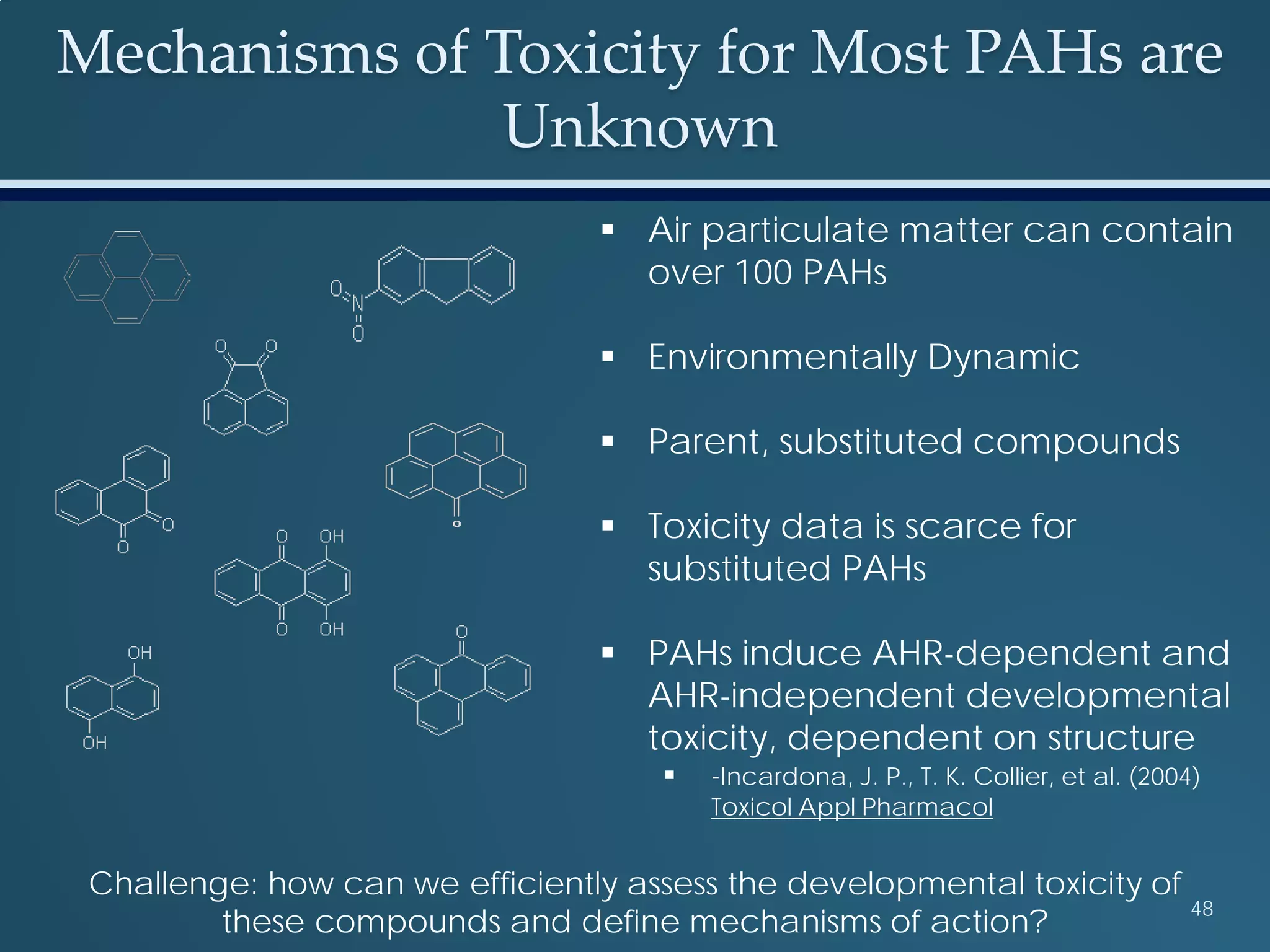
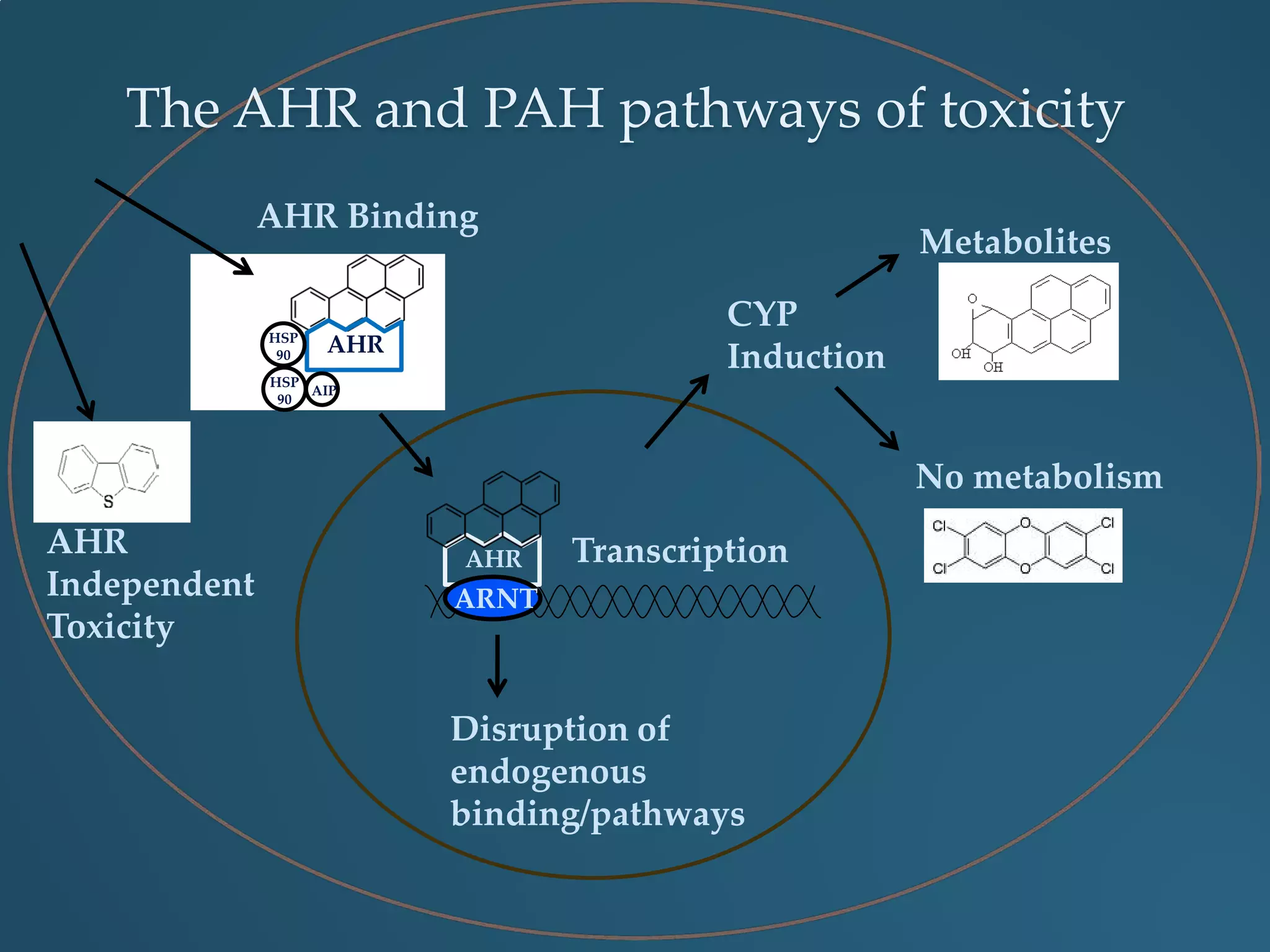
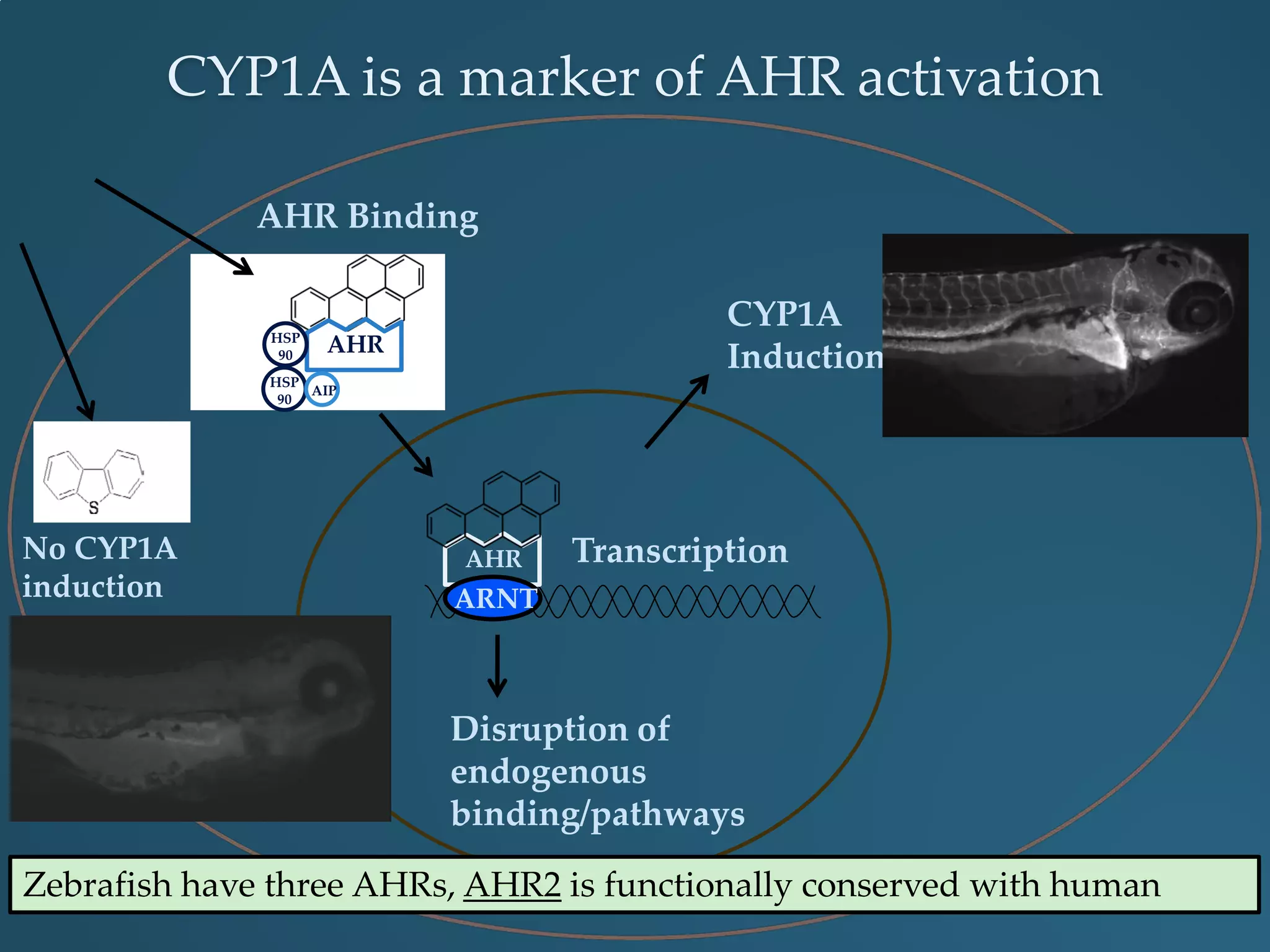
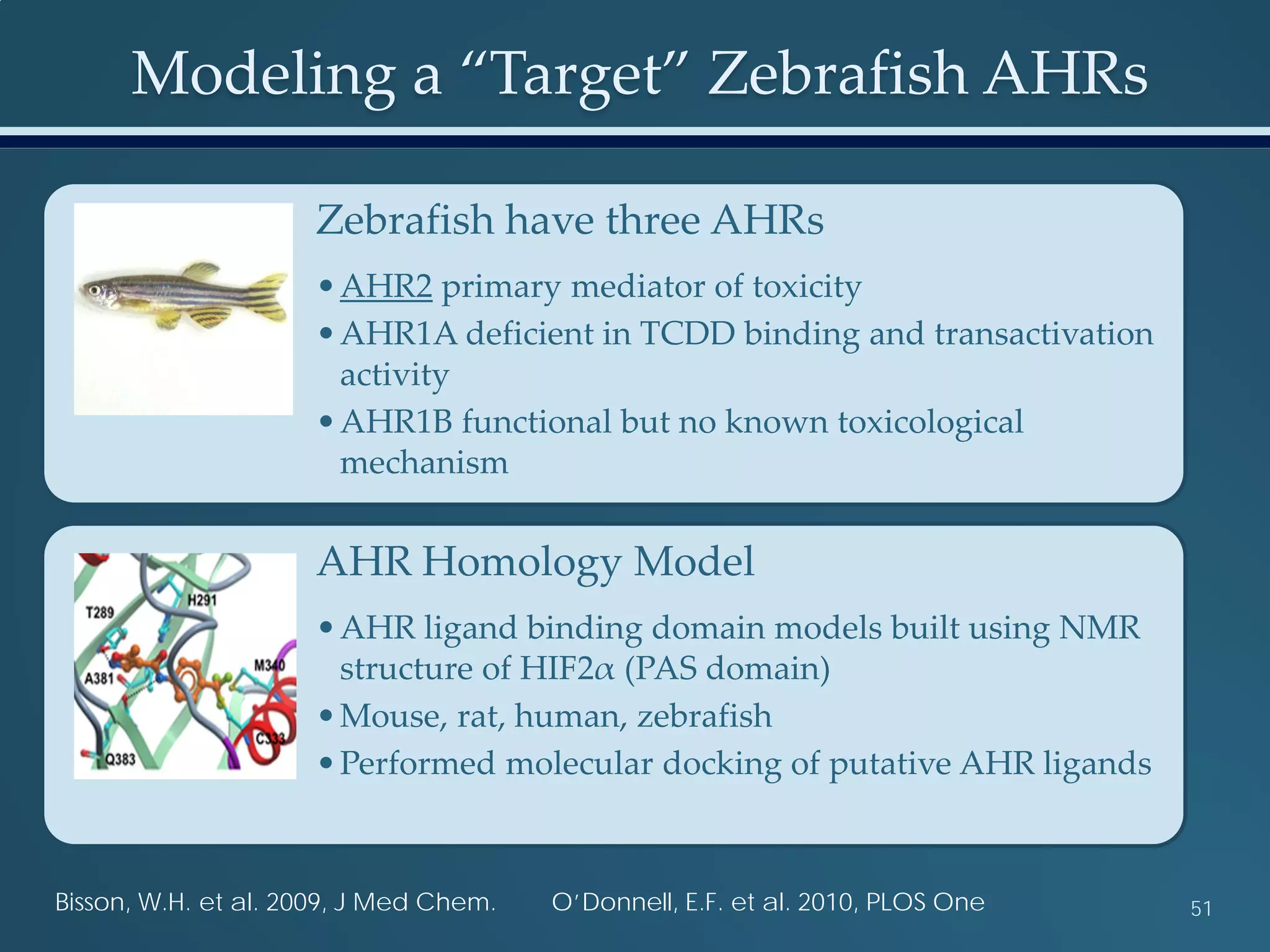
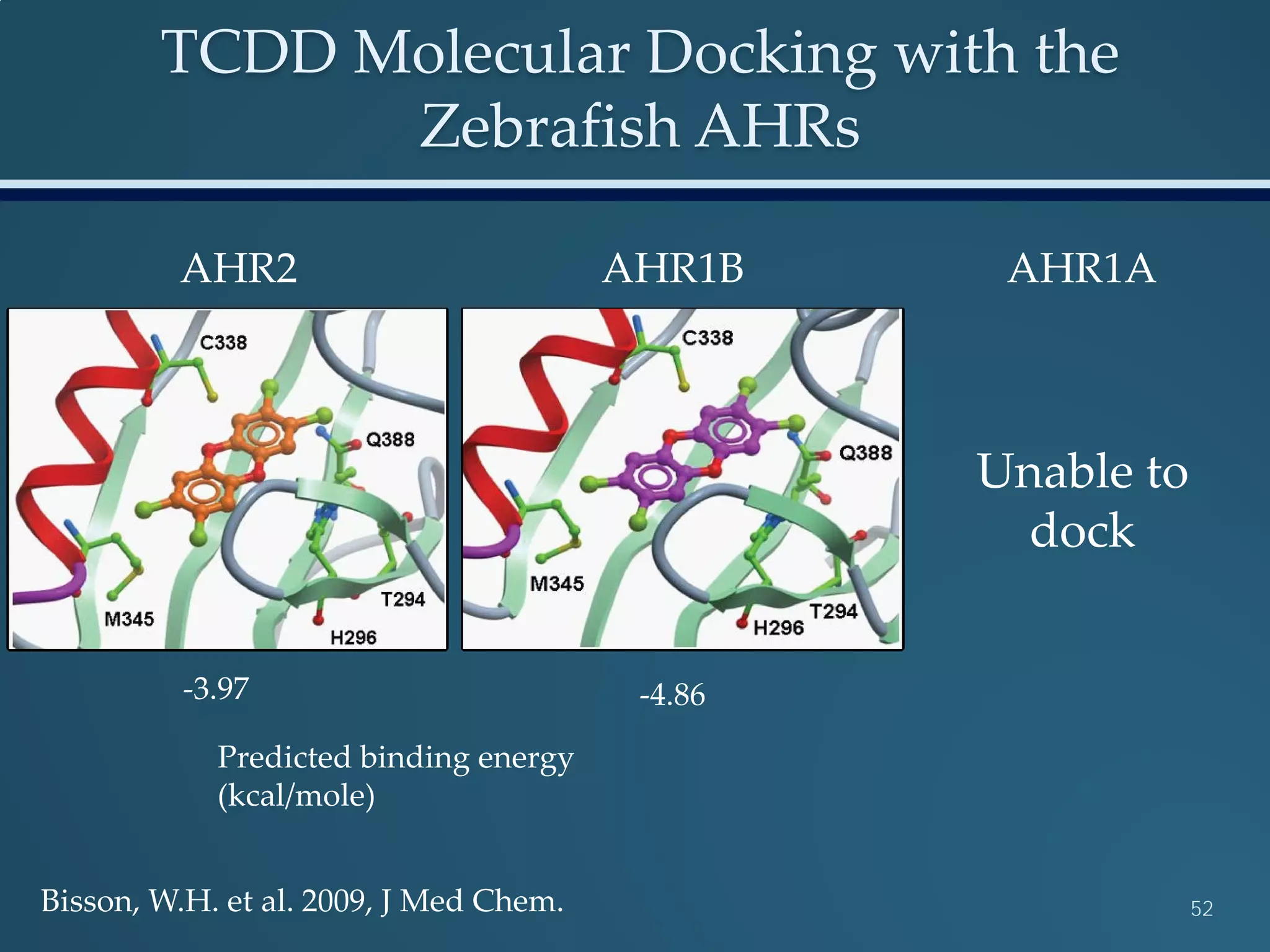
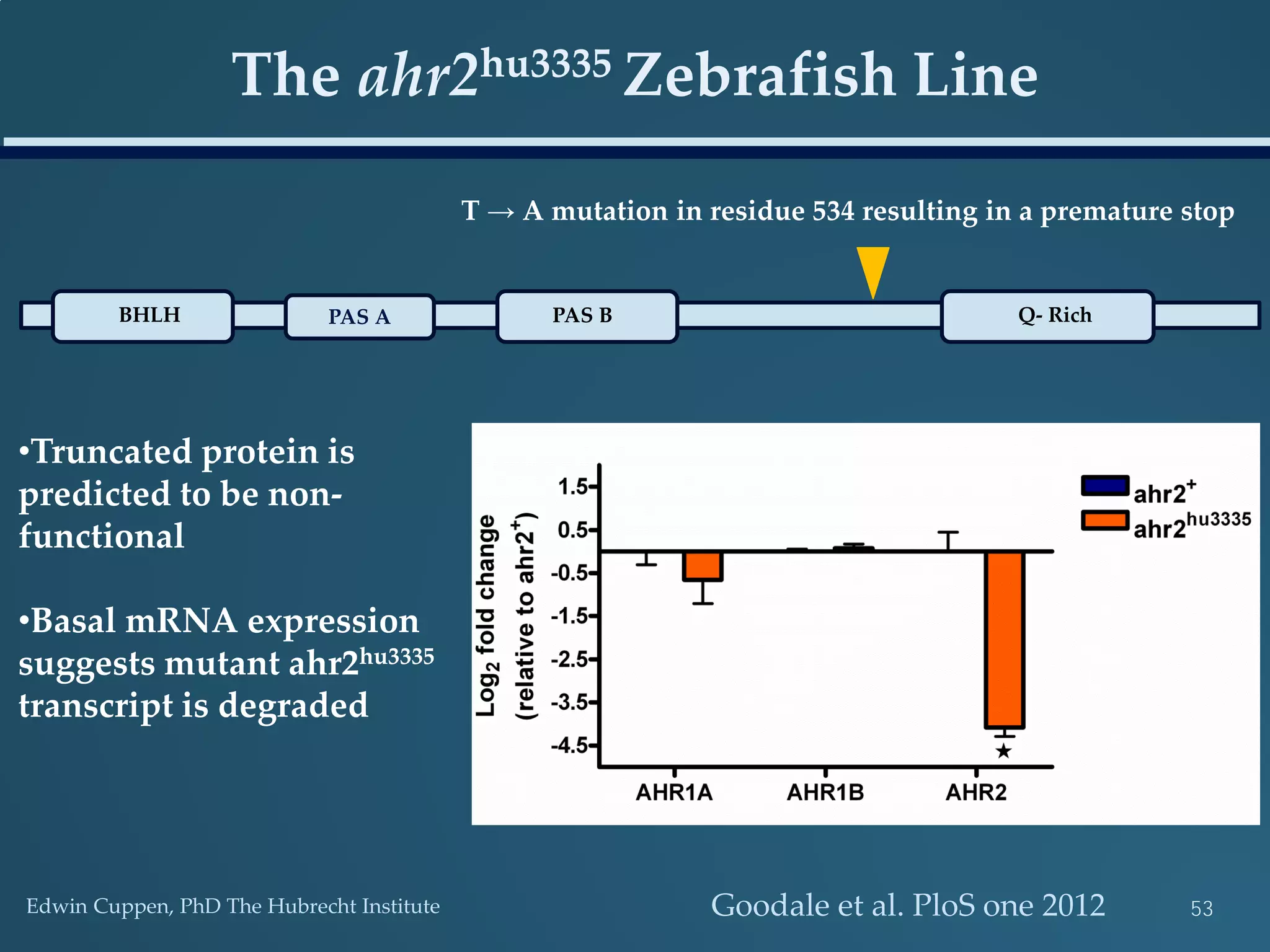
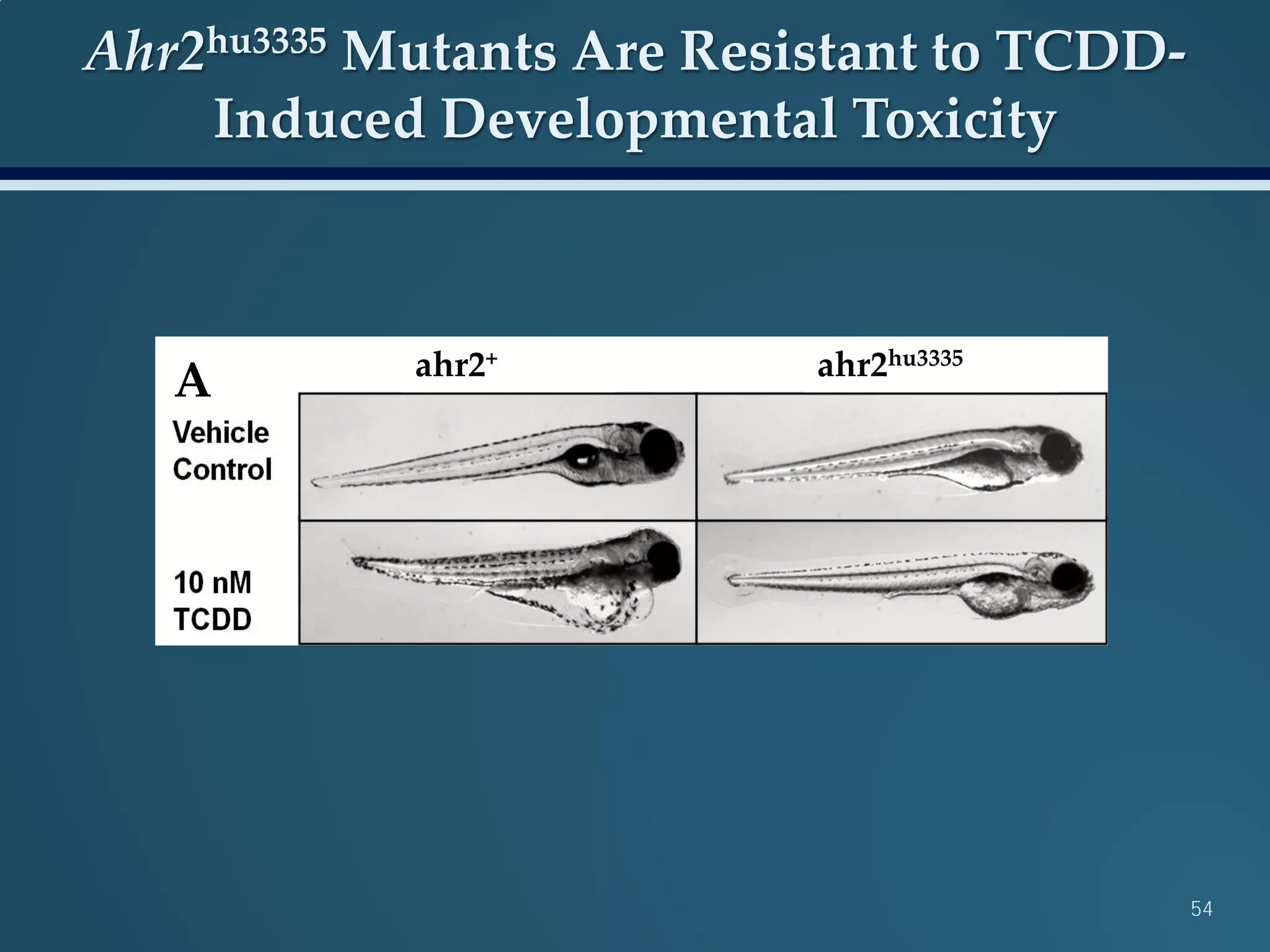
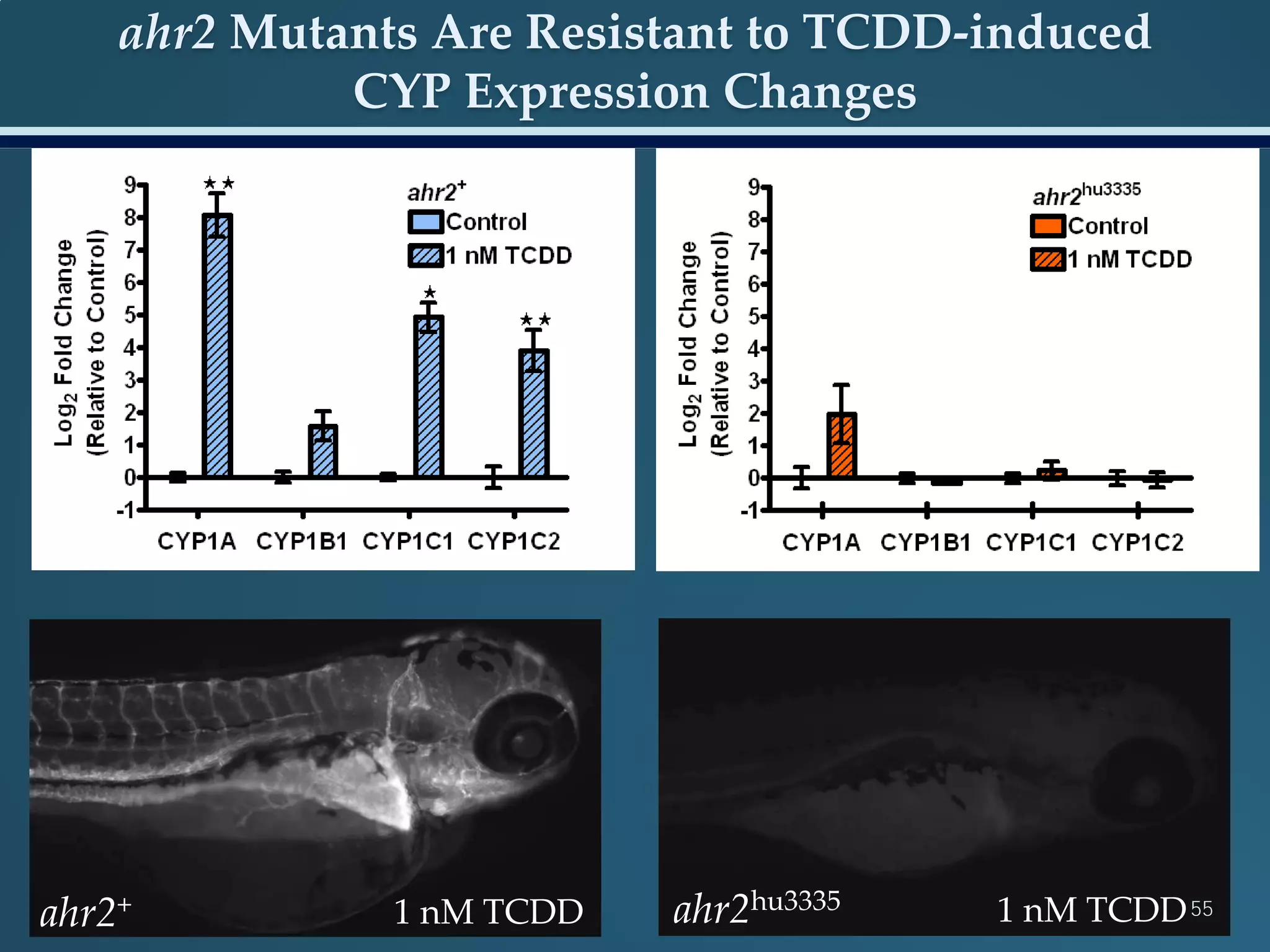
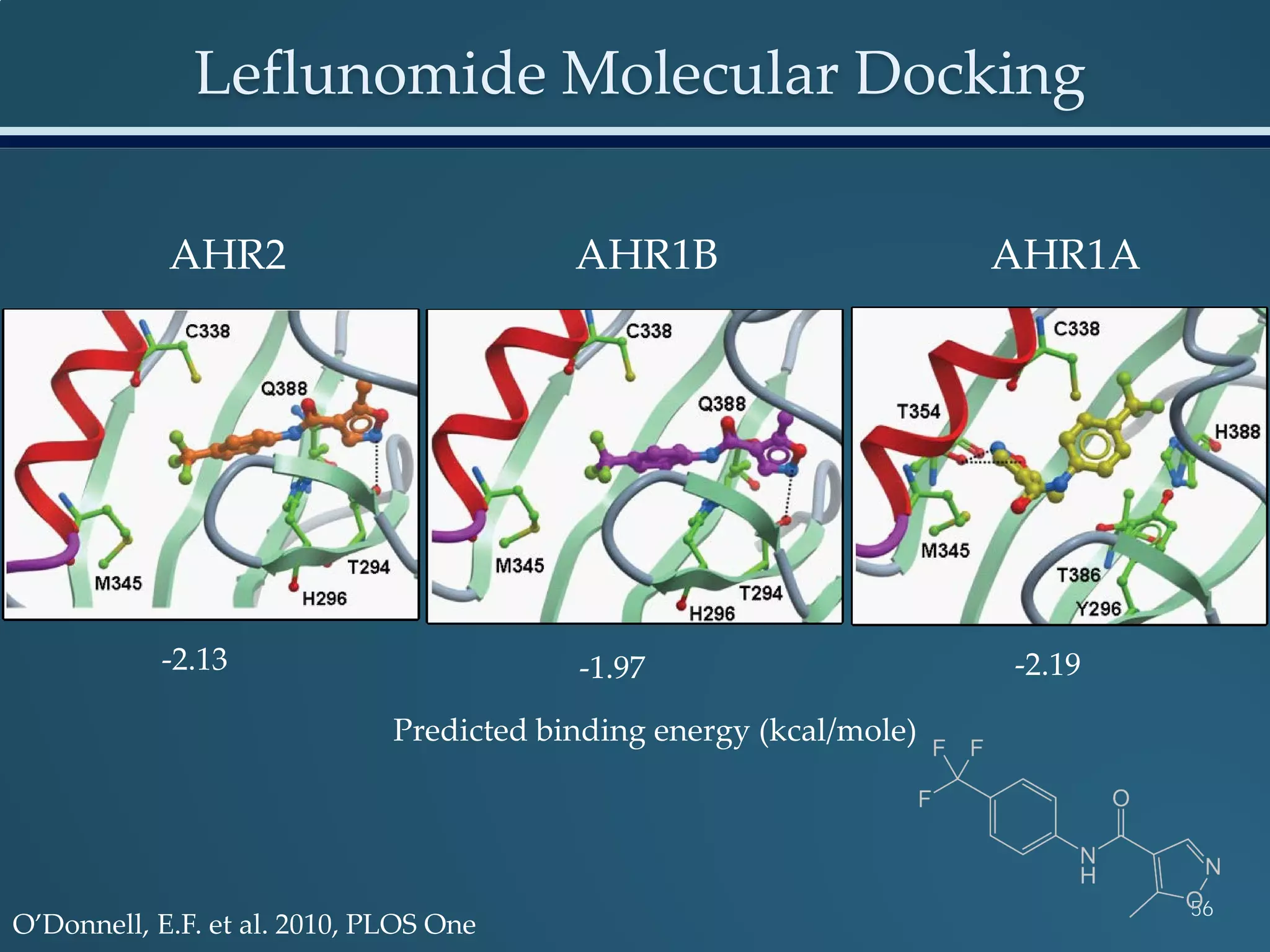
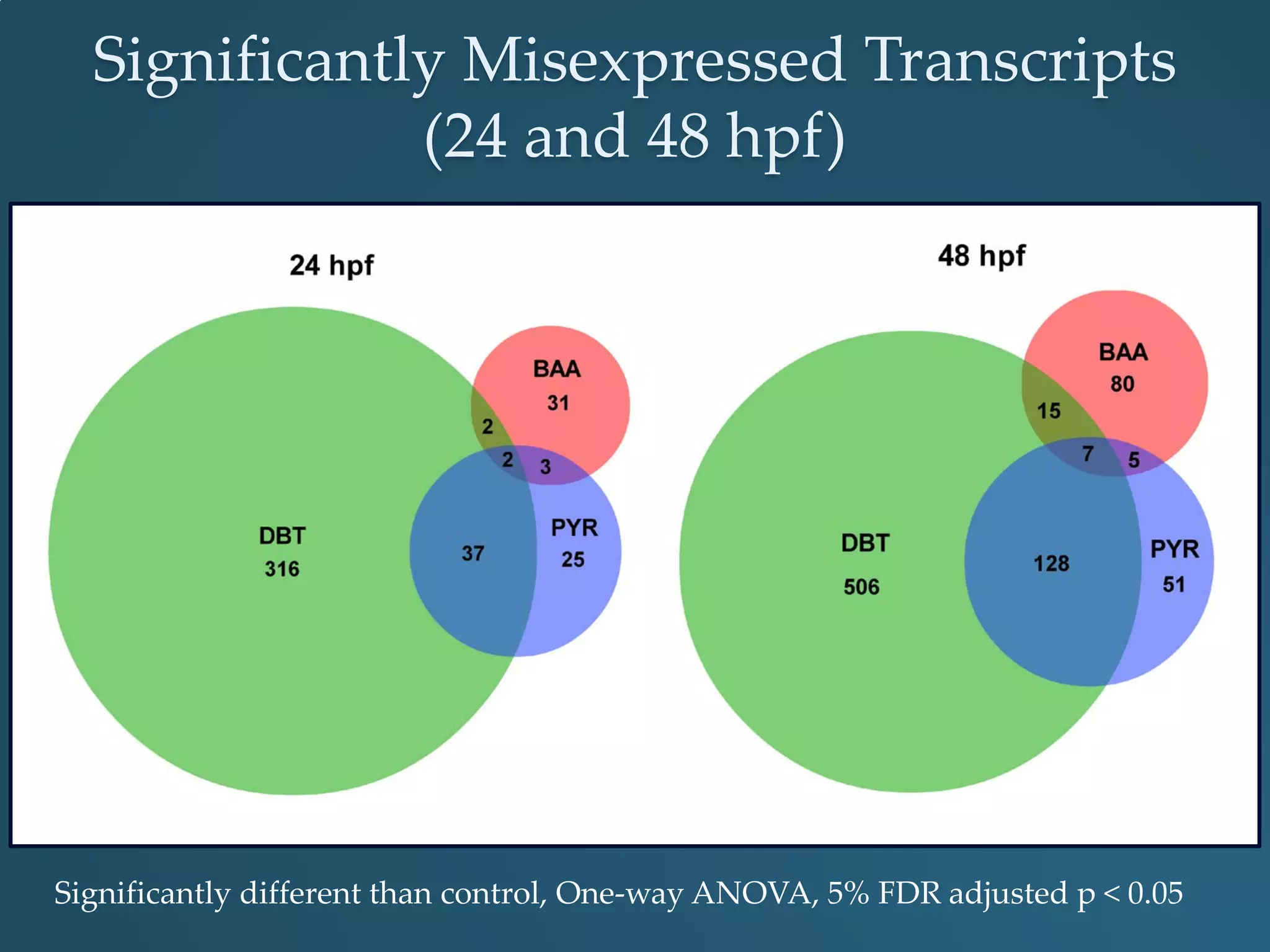
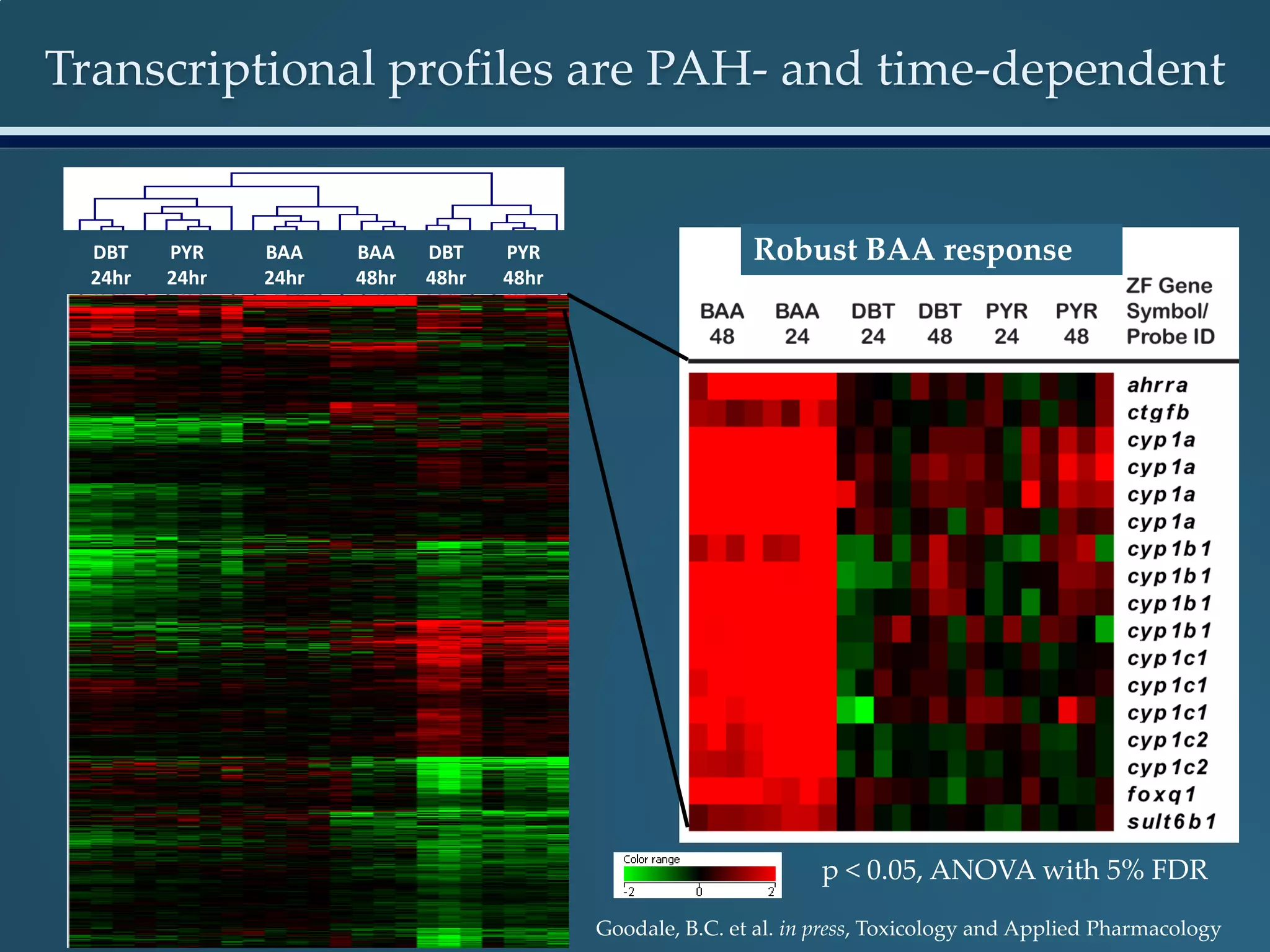
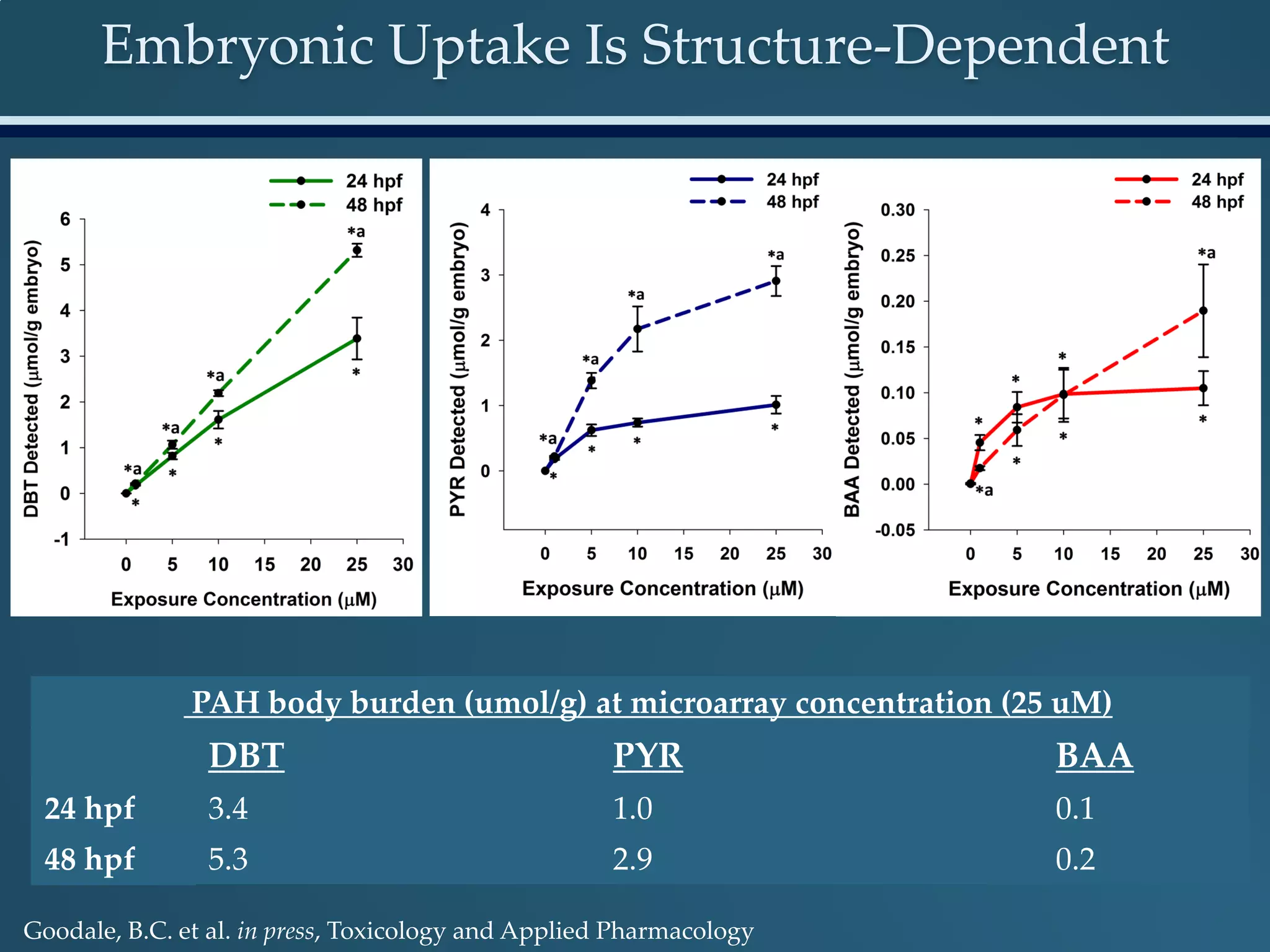
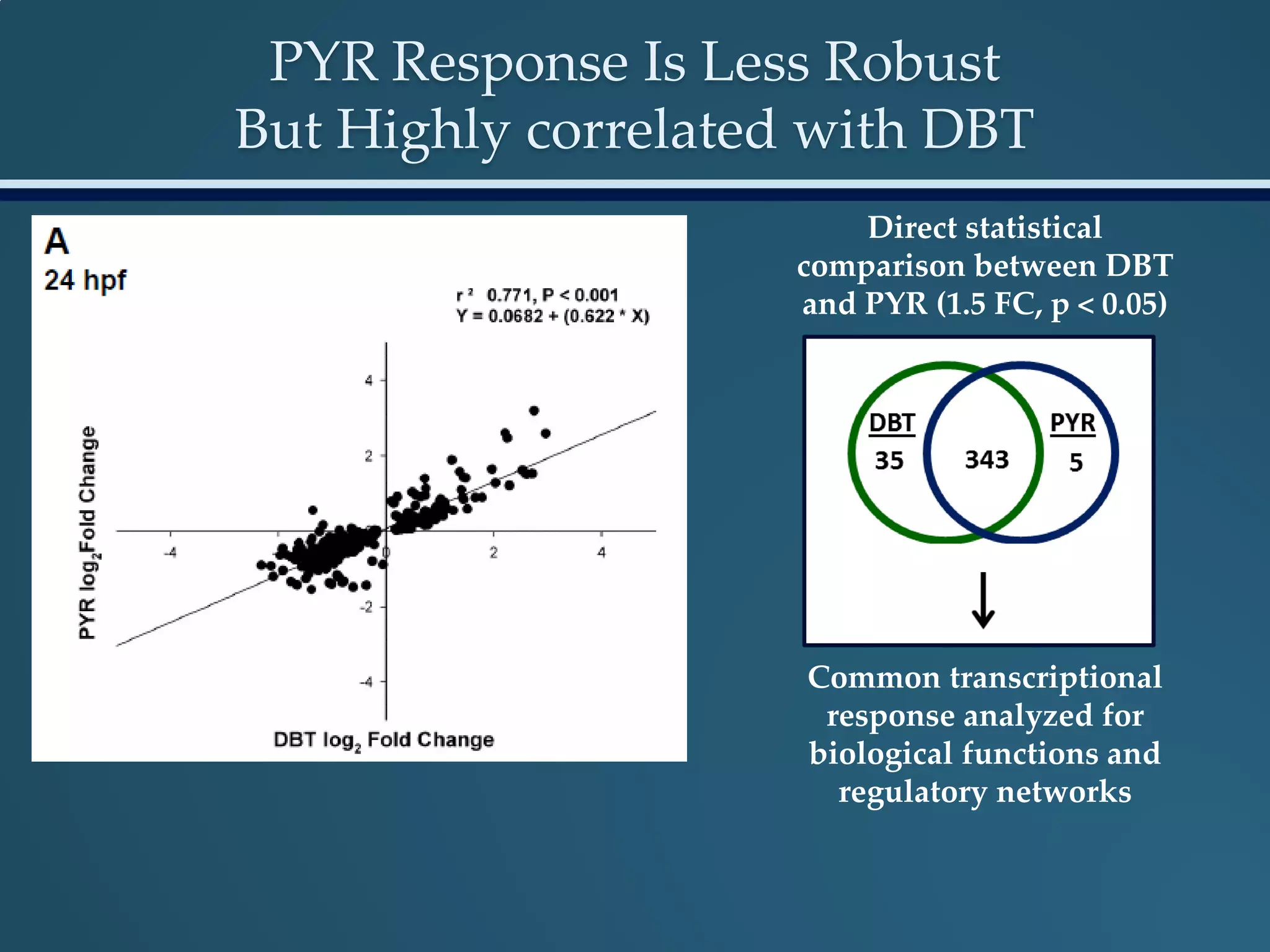
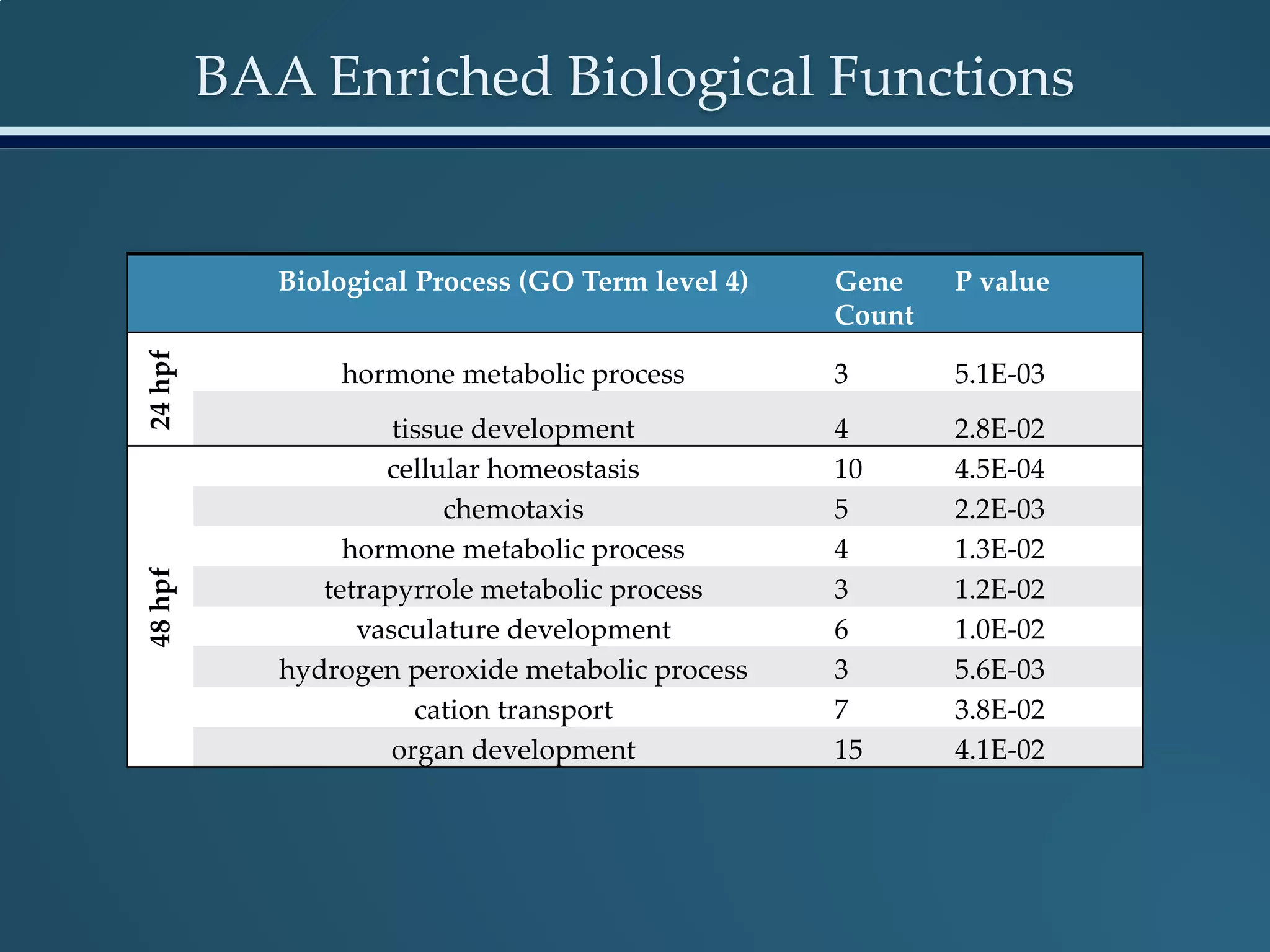
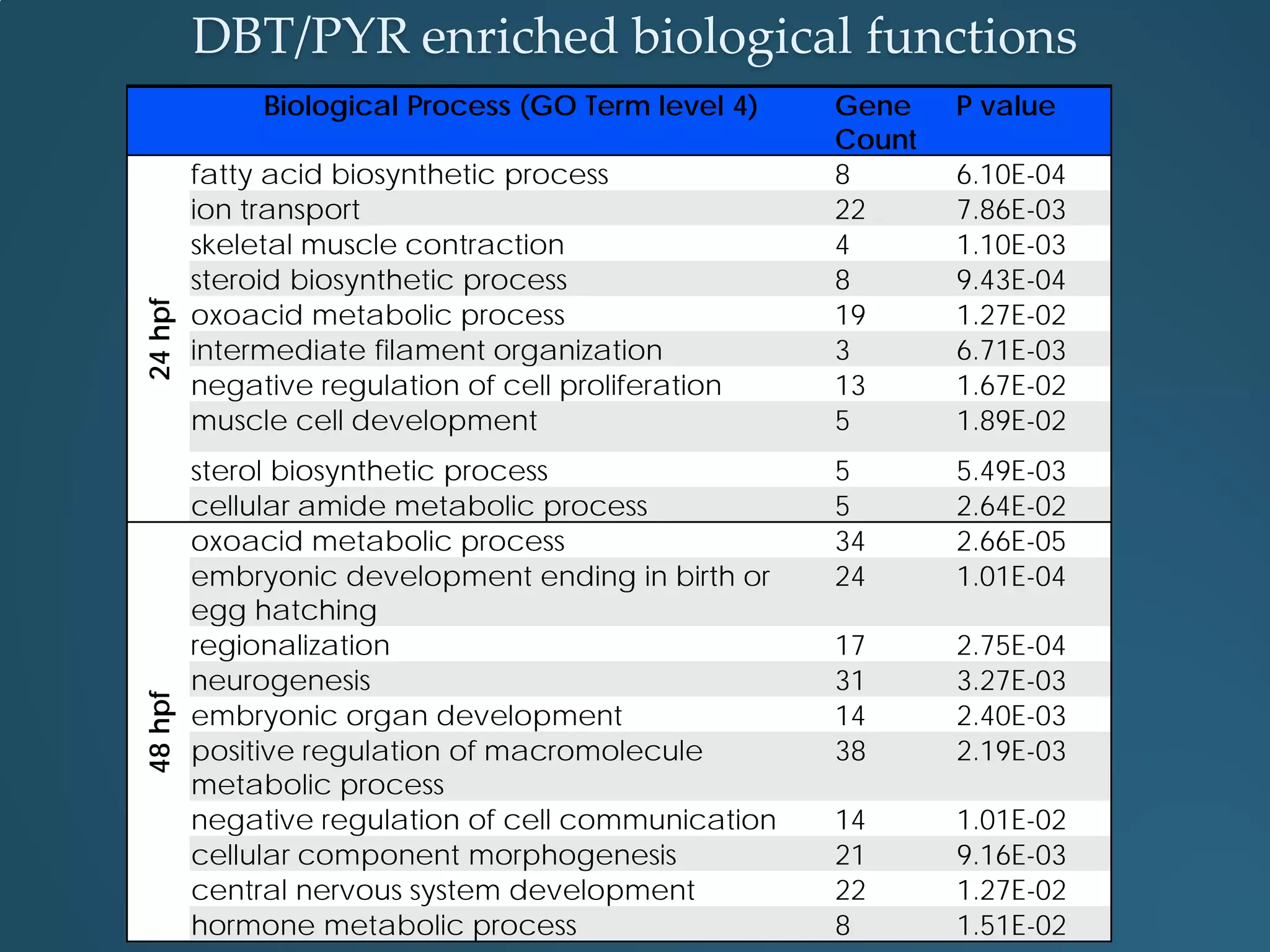
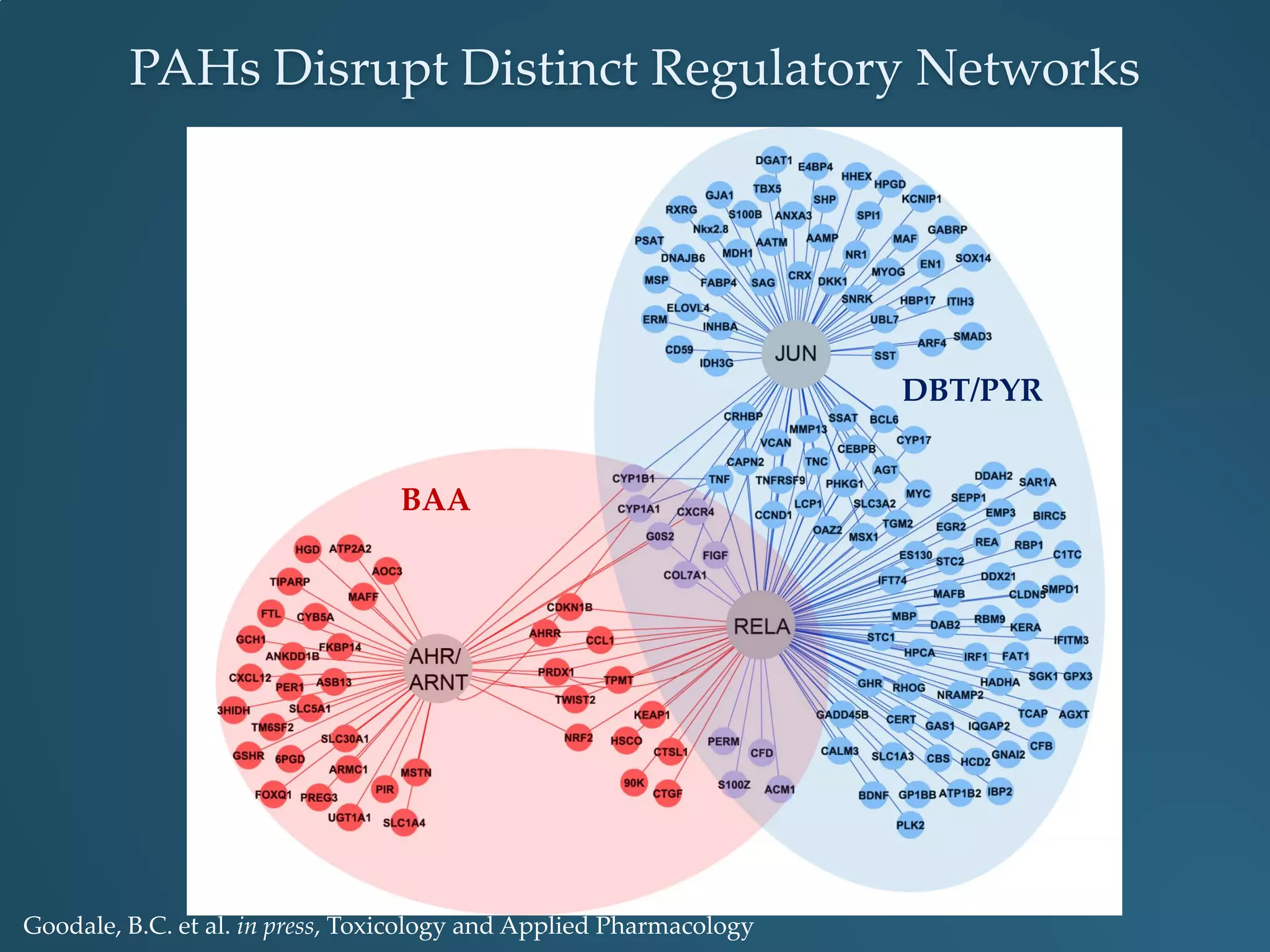
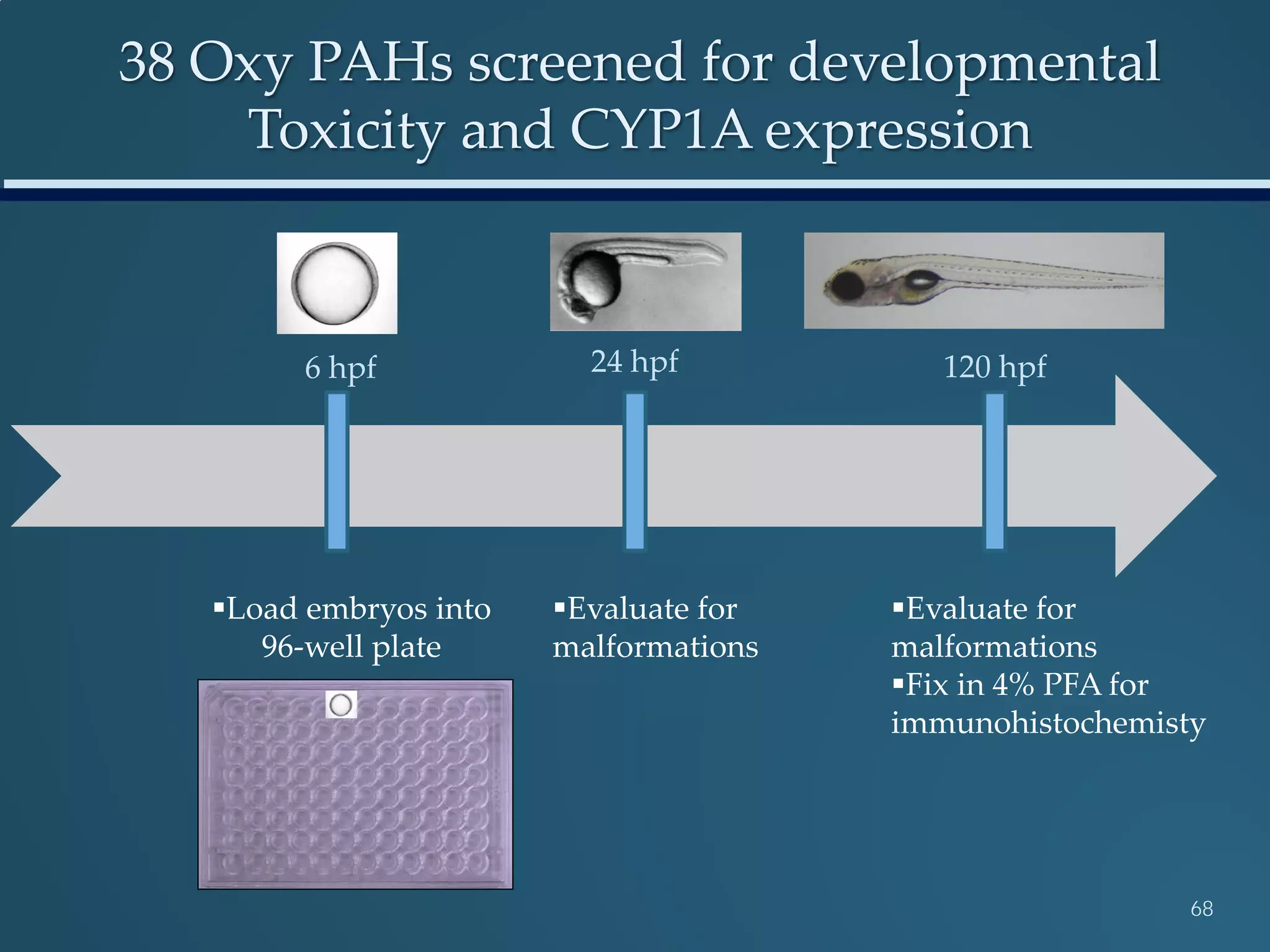
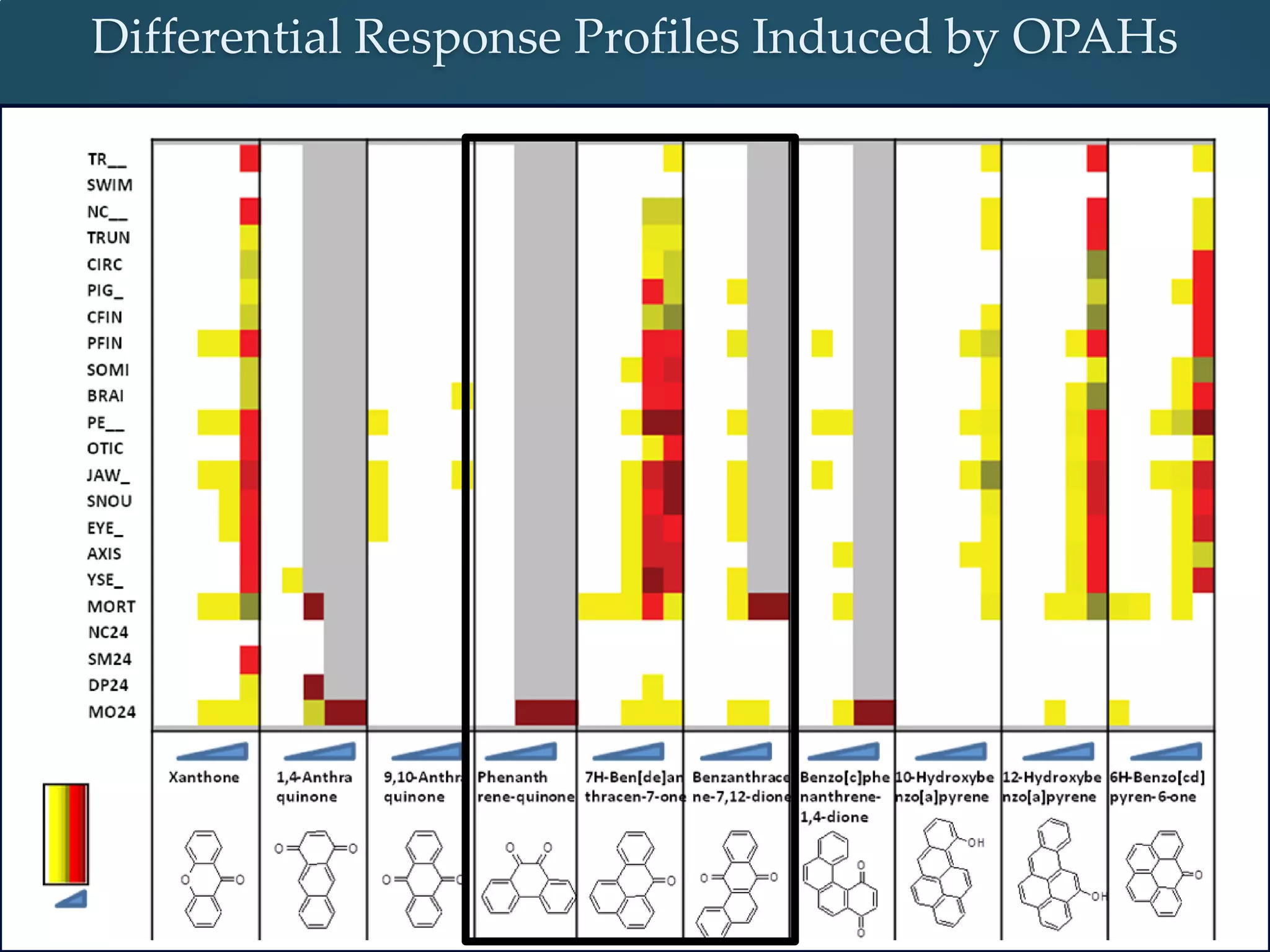
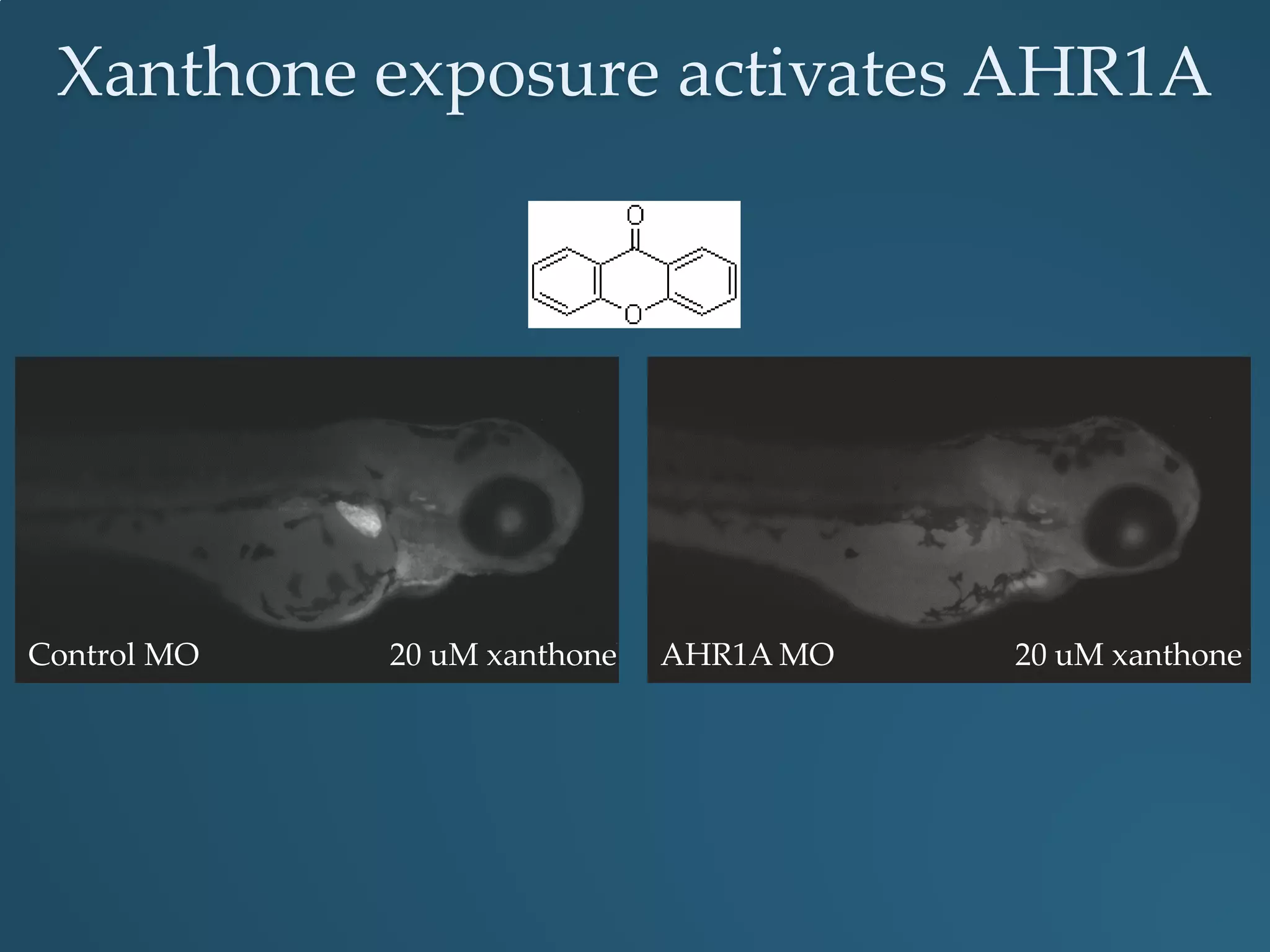
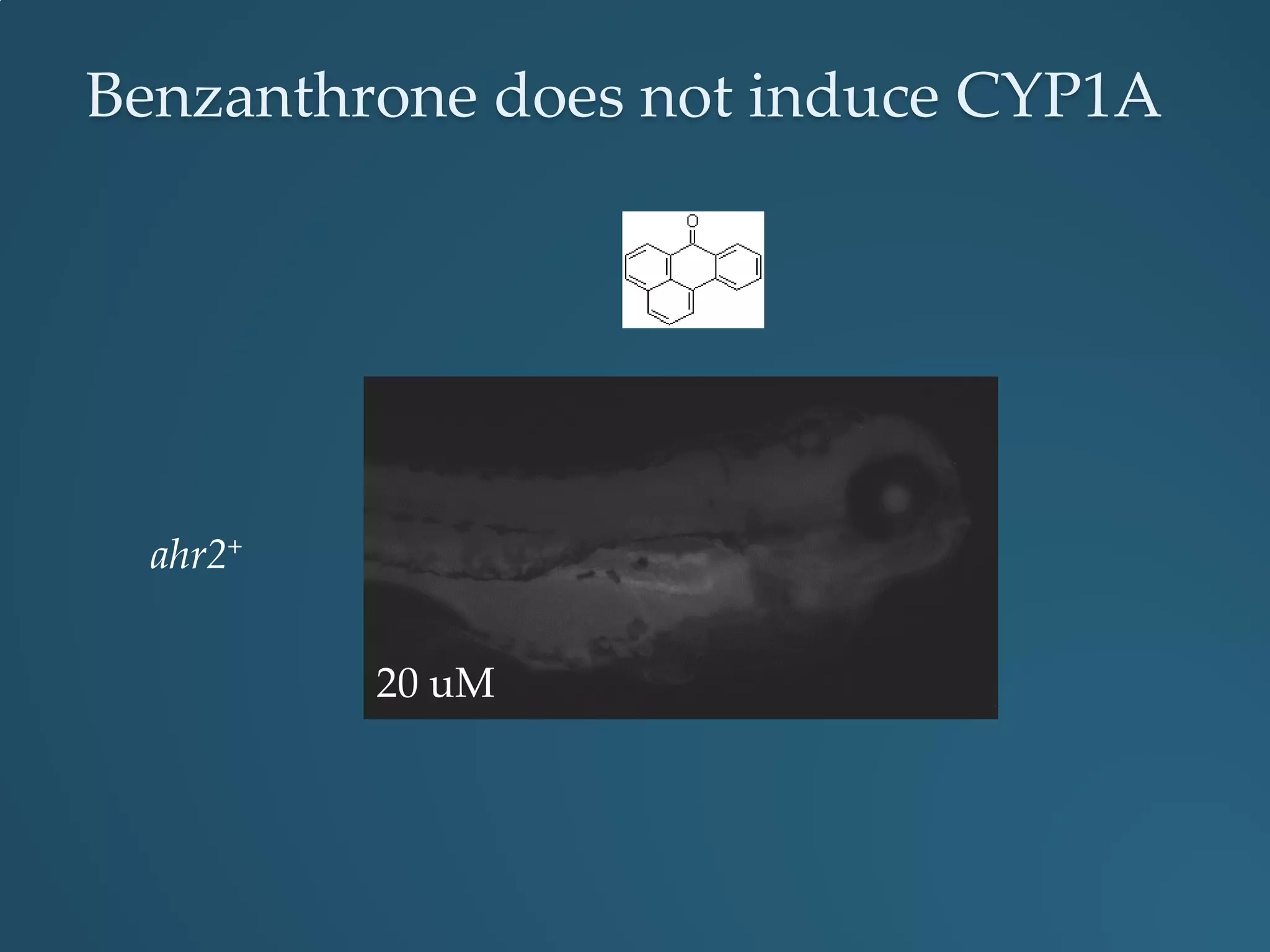
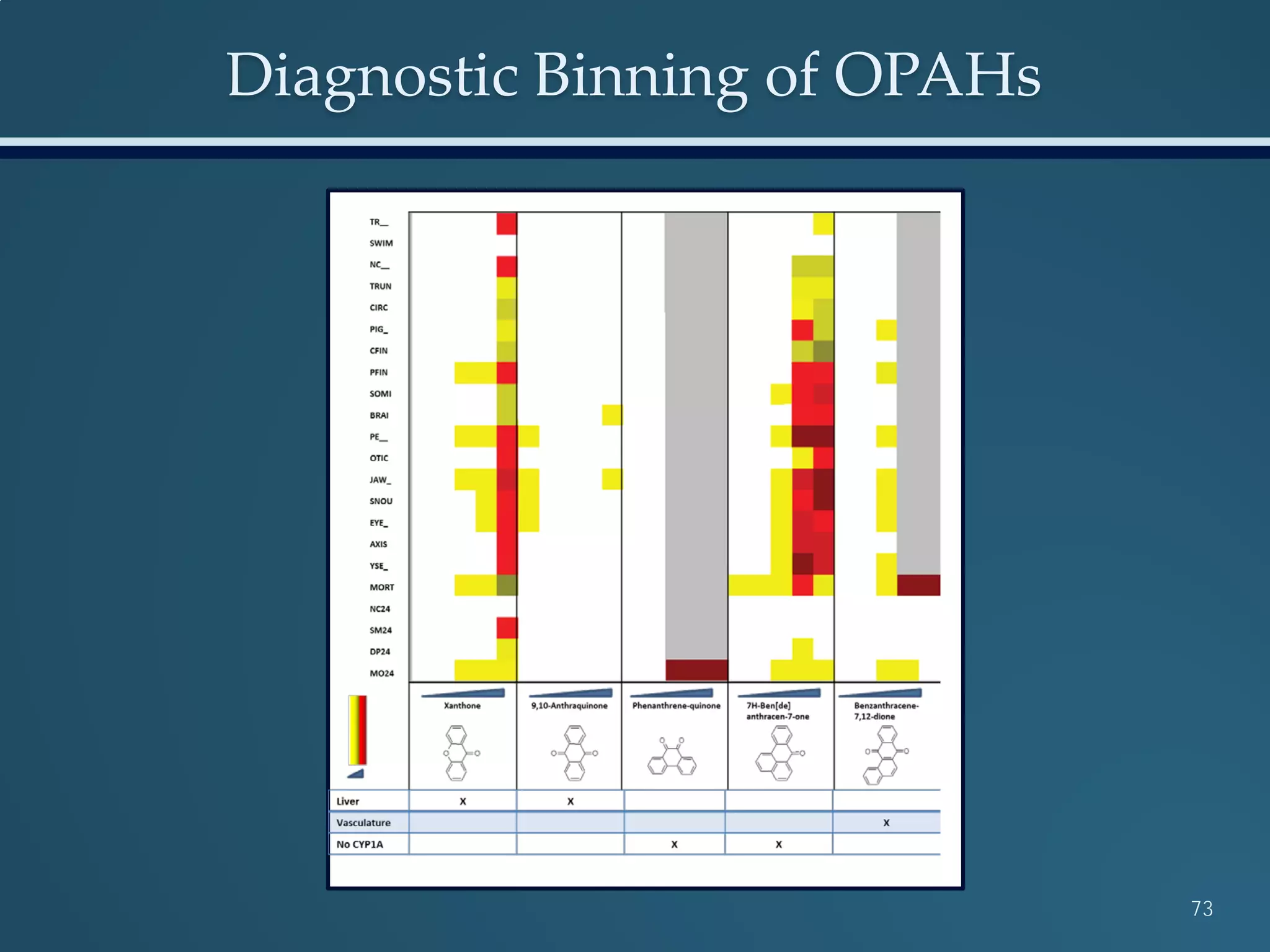
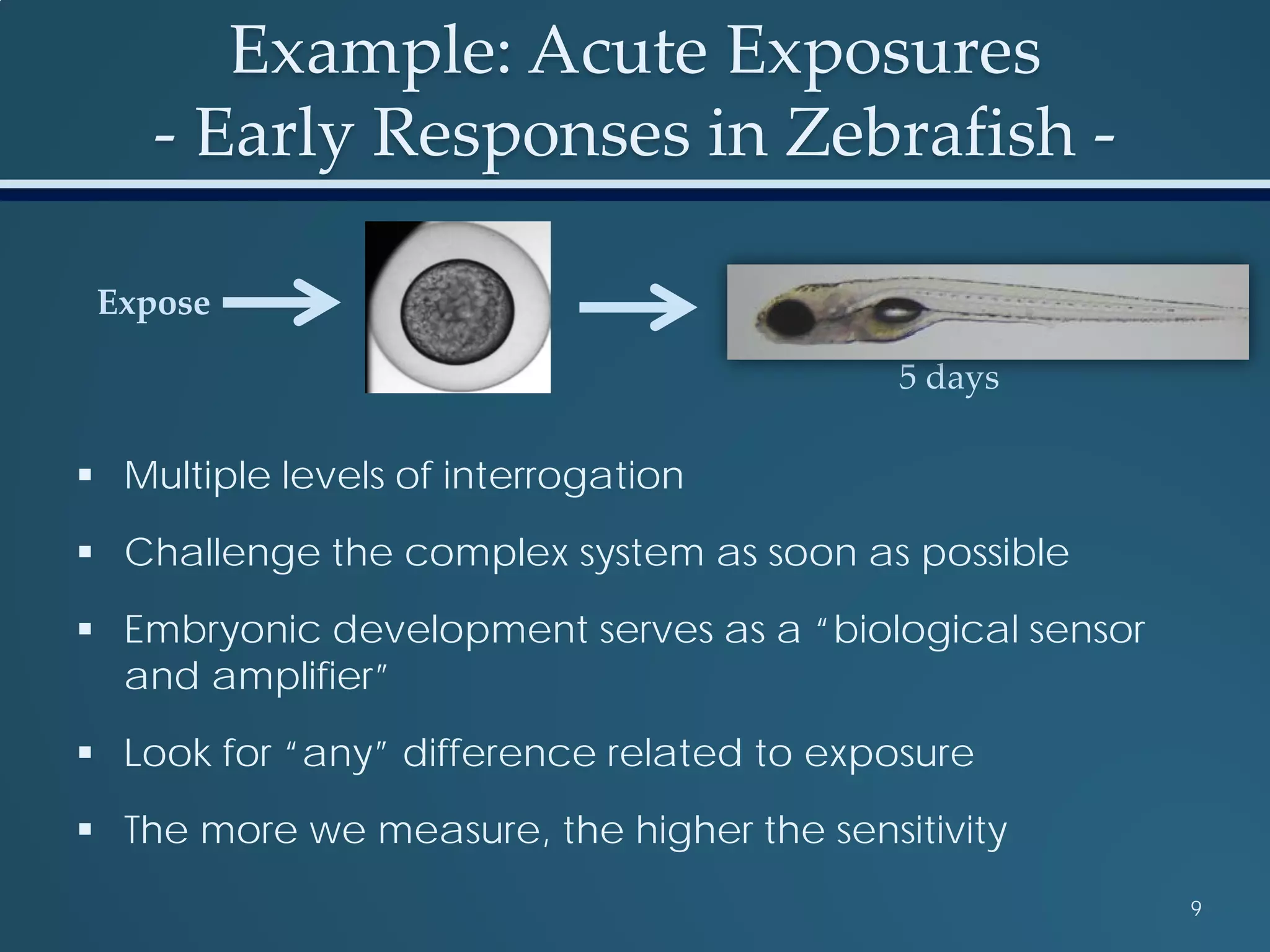
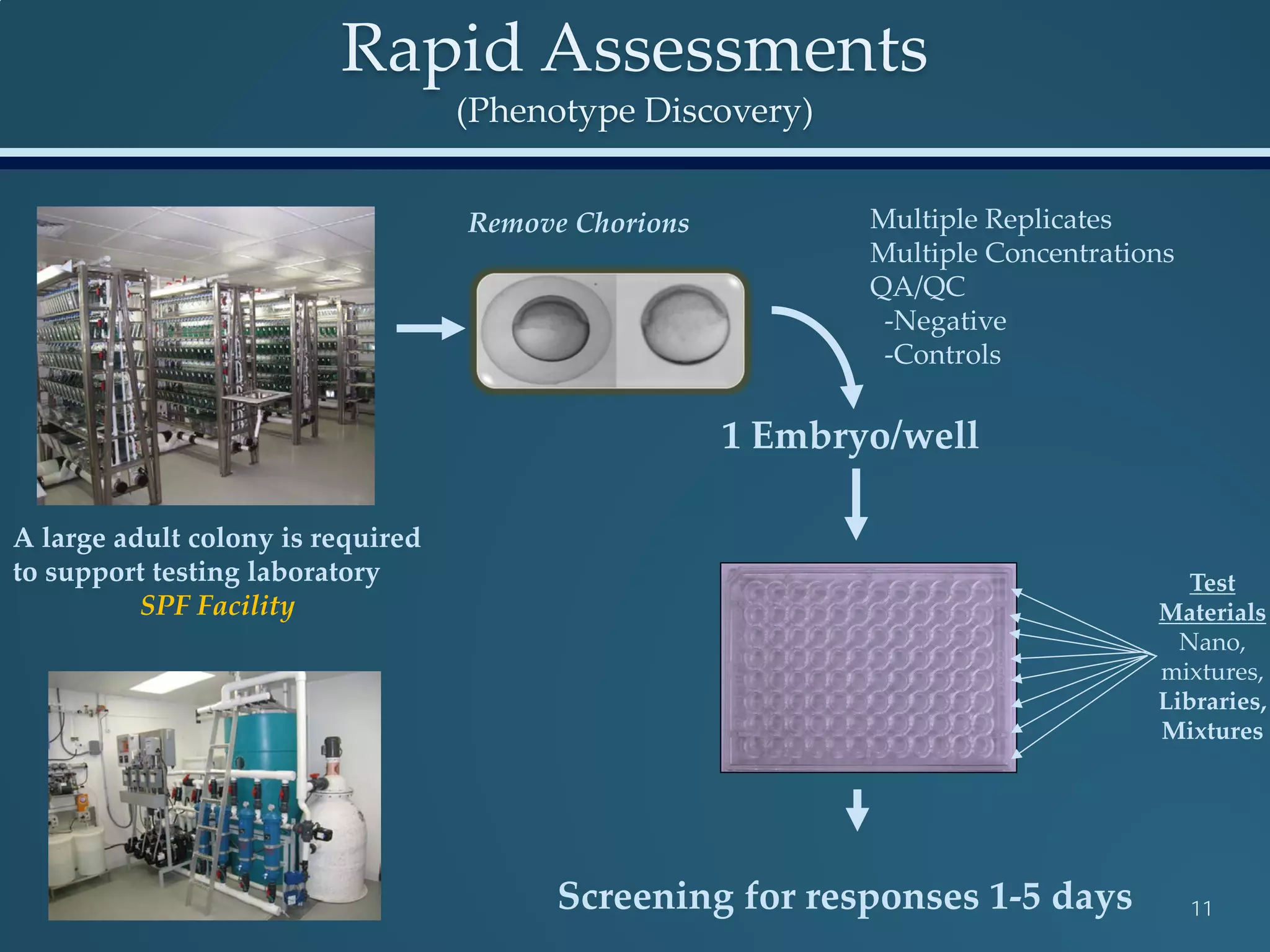
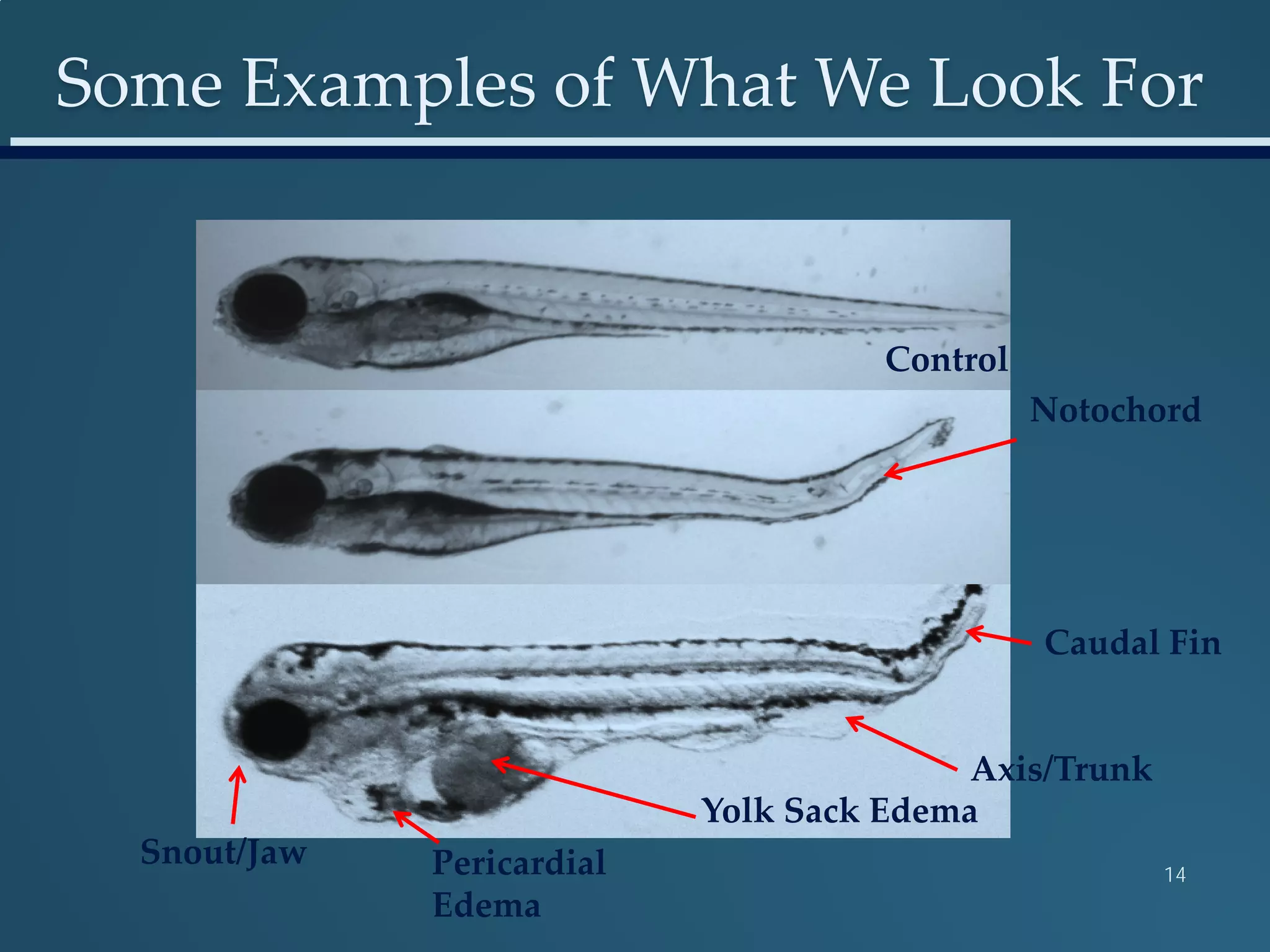
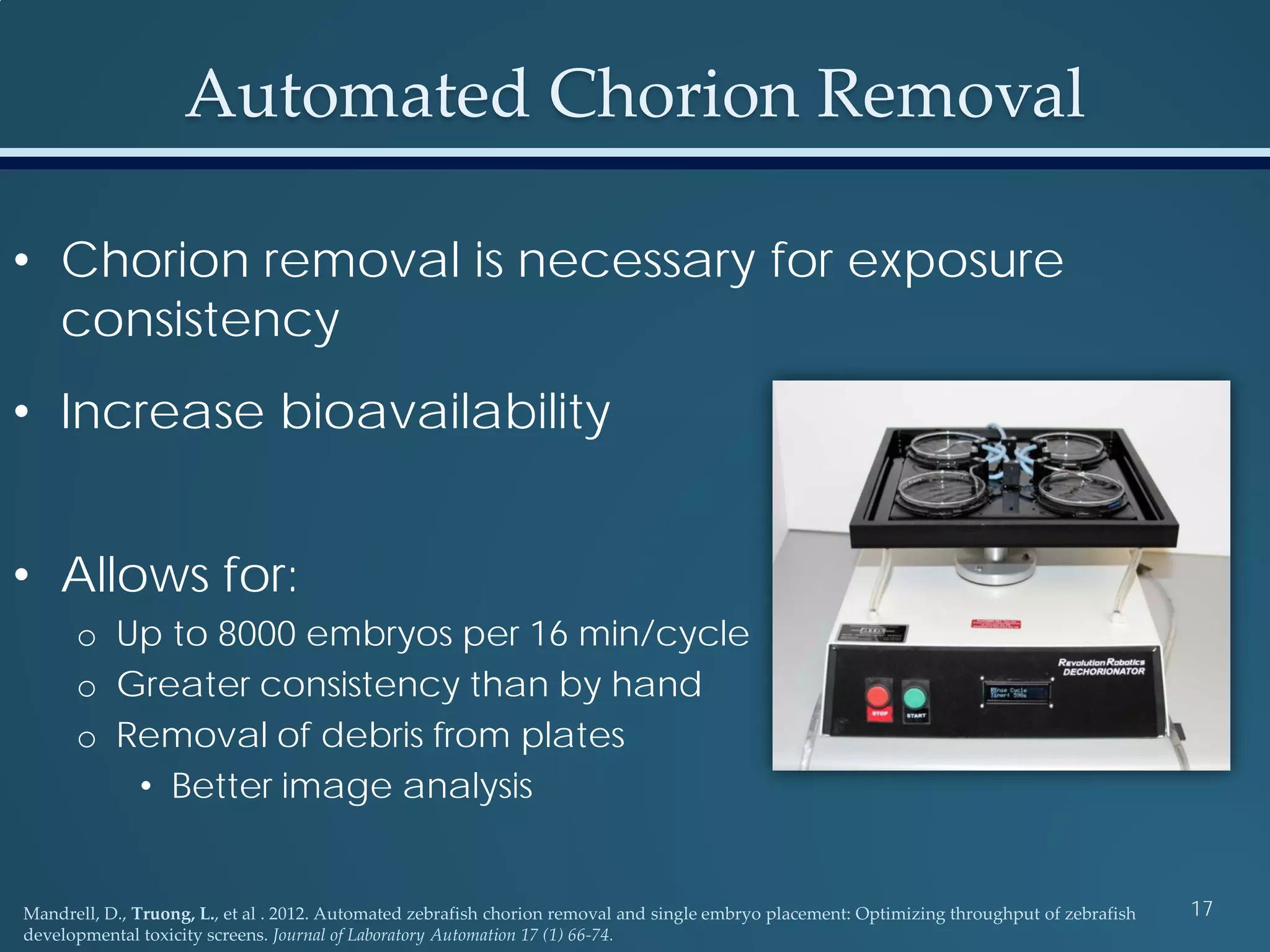
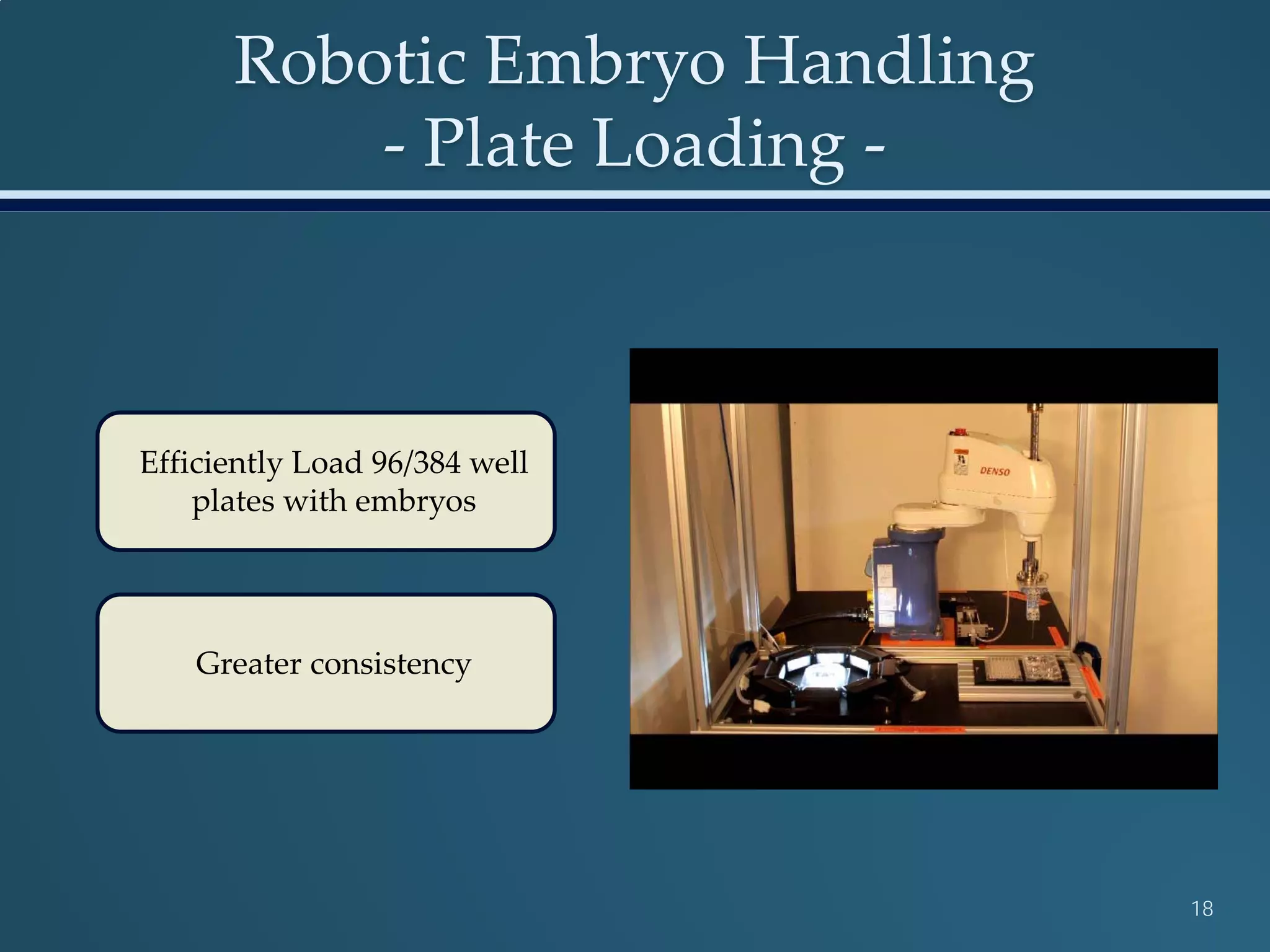
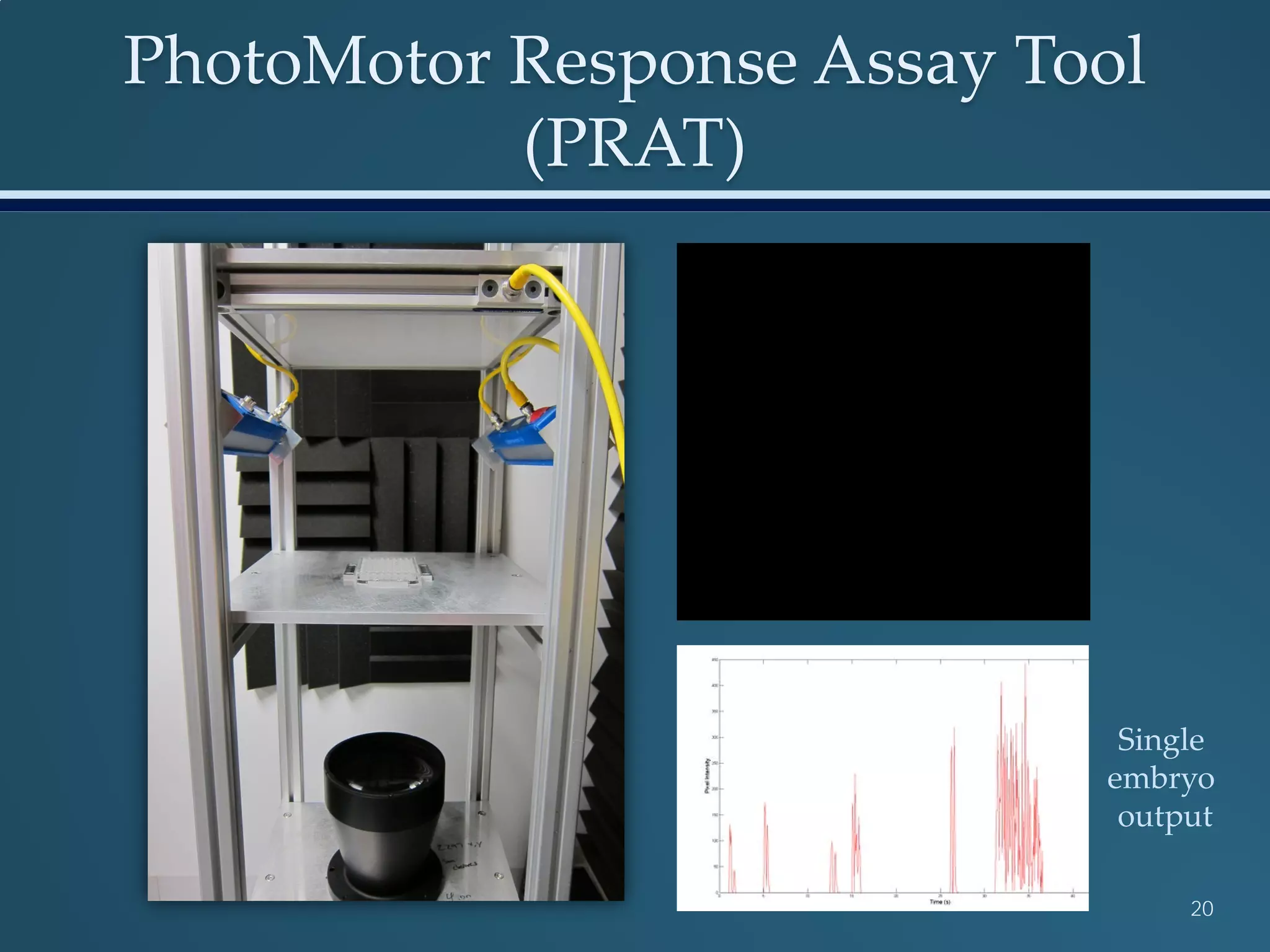
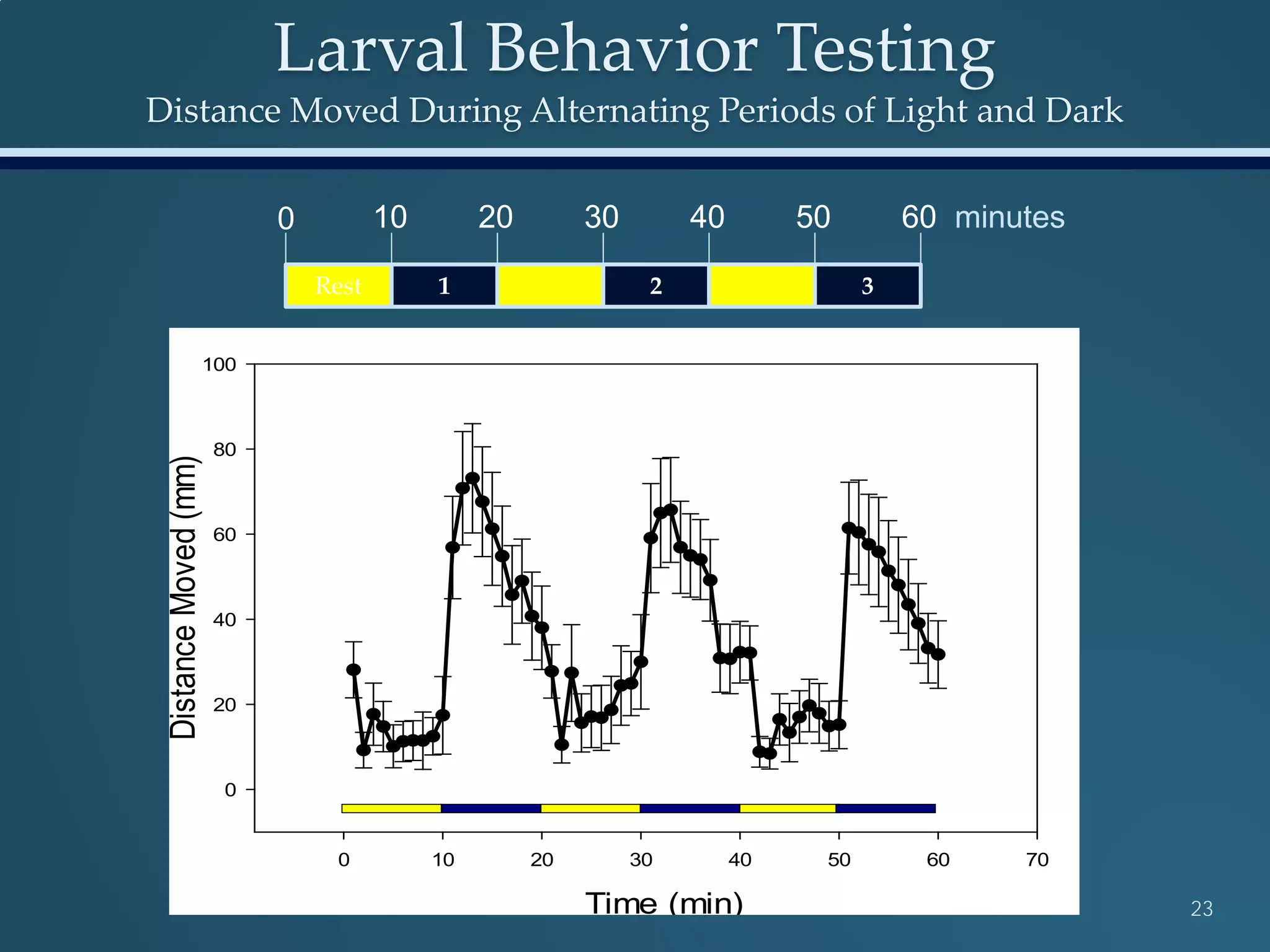
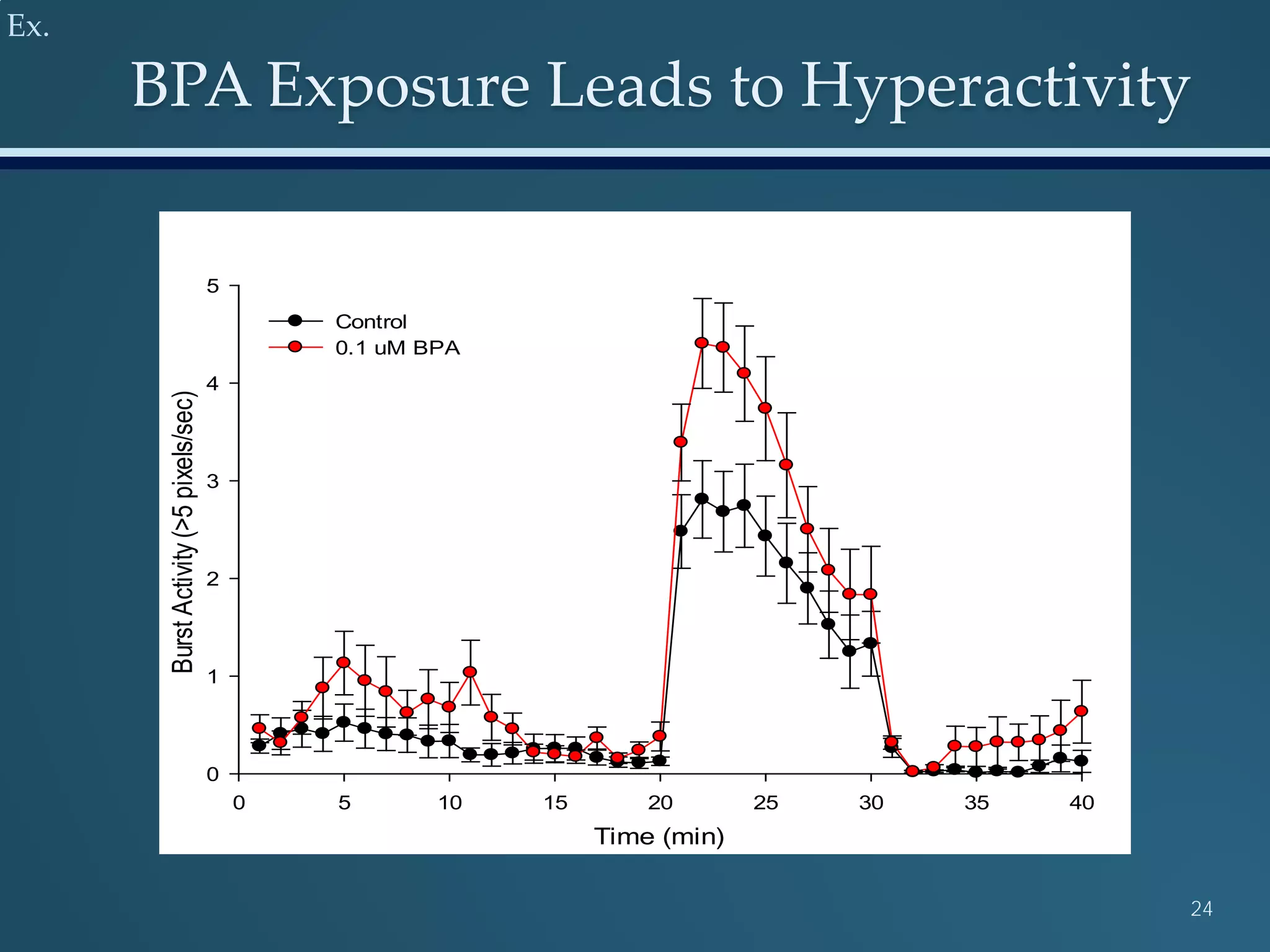
This document discusses the rapid in vivo assessment of bioactivity in zebrafish for predictive toxicology, detailing methods for evaluating chemical exposures and their effects on embryonic development. It emphasizes the importance of high-throughput screening and automation to increase efficiency in assessing developmental toxicity and understanding molecular responses to environmental pollutants. The research is supported by findings related to polycyclic aromatic hydrocarbons and various experimental designs used for behavioral and morphological assessments in zebrafish as a model organism.













![Fertilization 6 h 24 h (1 day)
Chemical Exposure
120 h (5 day)
[uM]
Light Pulse Exposure
Behavioral Assessment Developmental Assessment
And Motor Responses
= 1060 unique chemicals
x 6 concentrations
x 32 biological (well)
replicates
Integrated Screening Approach for
Developmental and Neurotoxicity](https://image.slidesharecdn.com/tanguaycalepa-140507124233-phpapp01/75/Rapid-In-Vivo-Assessment-of-Bioactivity-in-Zebrafish-High-Content-Data-for-Predictive-Toxicology-26-2048.jpg)
![HTS: High Throughput Screening
1060 chemicals x 18 endpoints
Analysis considerations
• Correlation structure
• Global patterns and “hit”
distributions
• Chemical property covariates
• Relationship between mortality
endpoint (MORT) and other specific
endpoints
• Comparison to related datasets
Zebrafish 5dpf Development: Analysis
[Truong et al. Tox Sci (2014)]](https://image.slidesharecdn.com/tanguaycalepa-140507124233-phpapp01/75/Rapid-In-Vivo-Assessment-of-Bioactivity-in-Zebrafish-High-Content-Data-for-Predictive-Toxicology-27-2048.jpg)