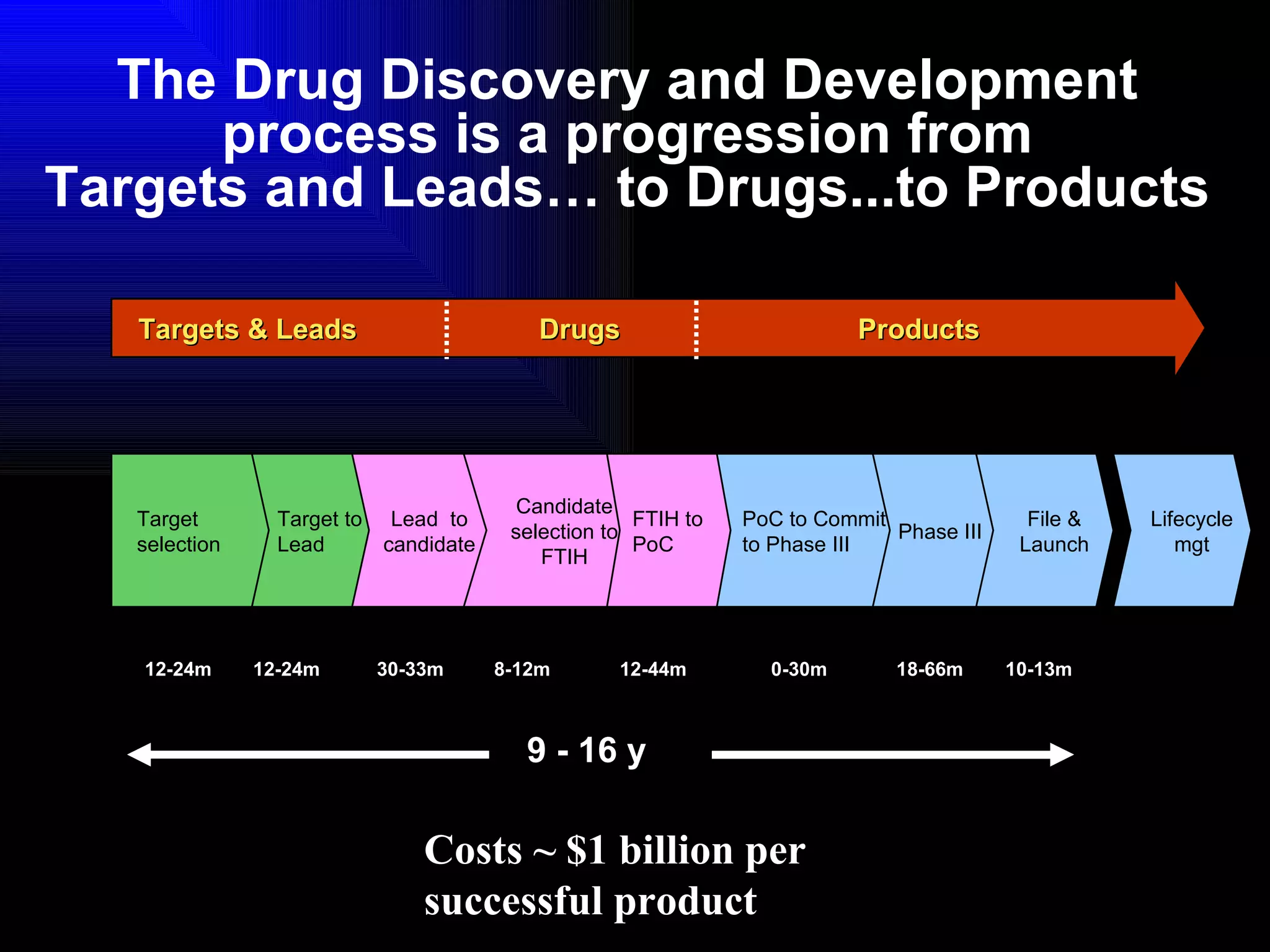
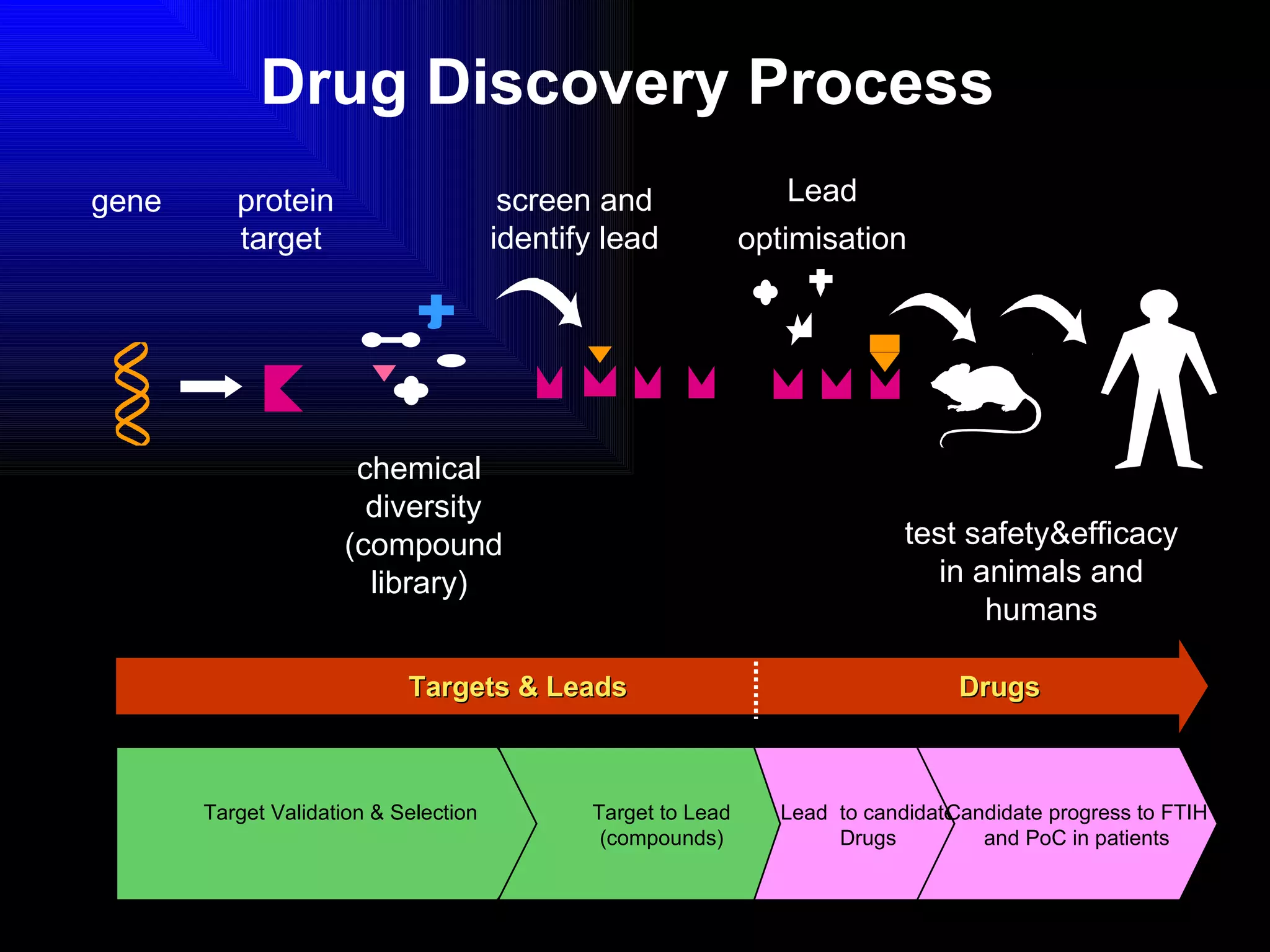
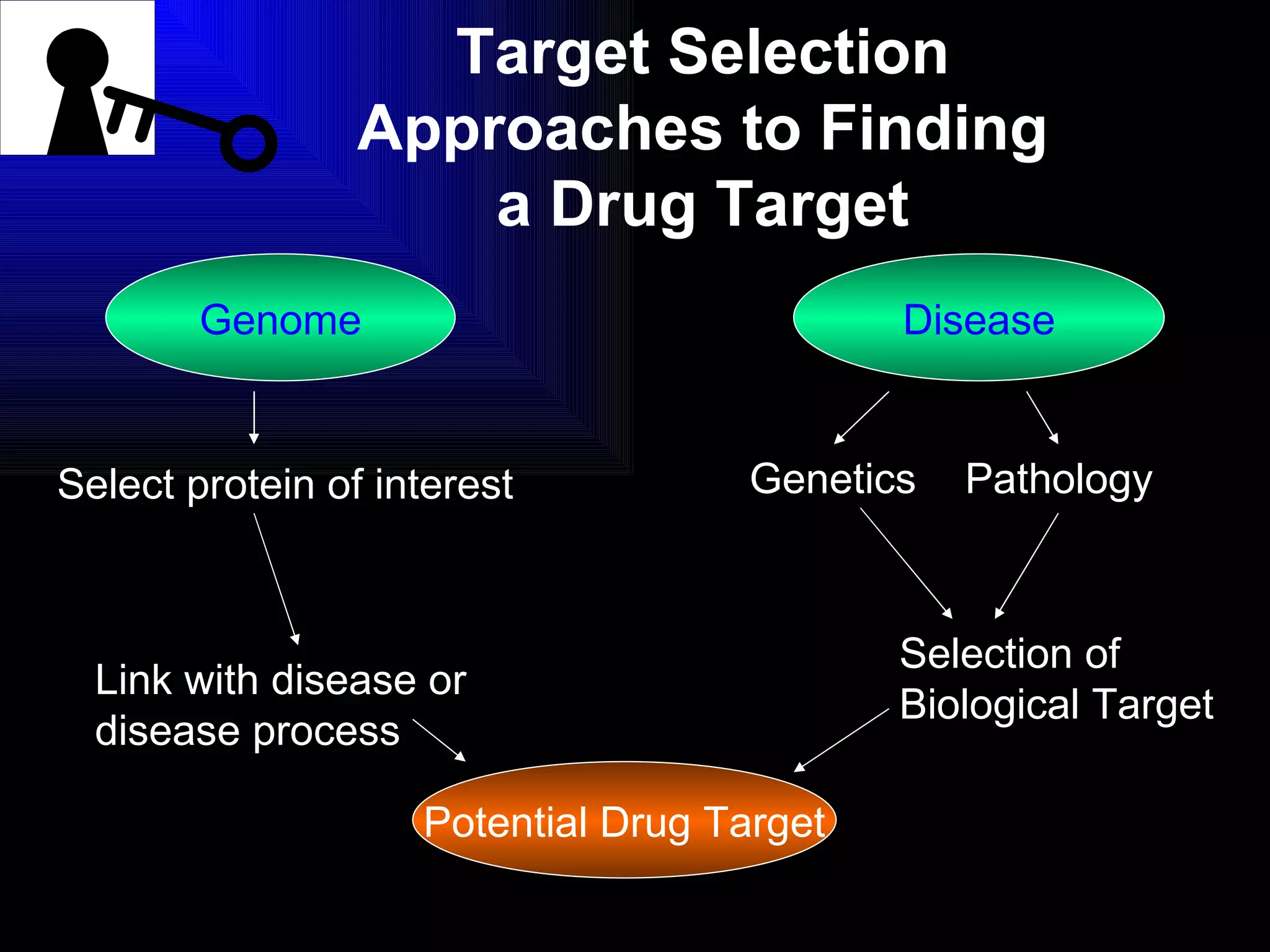
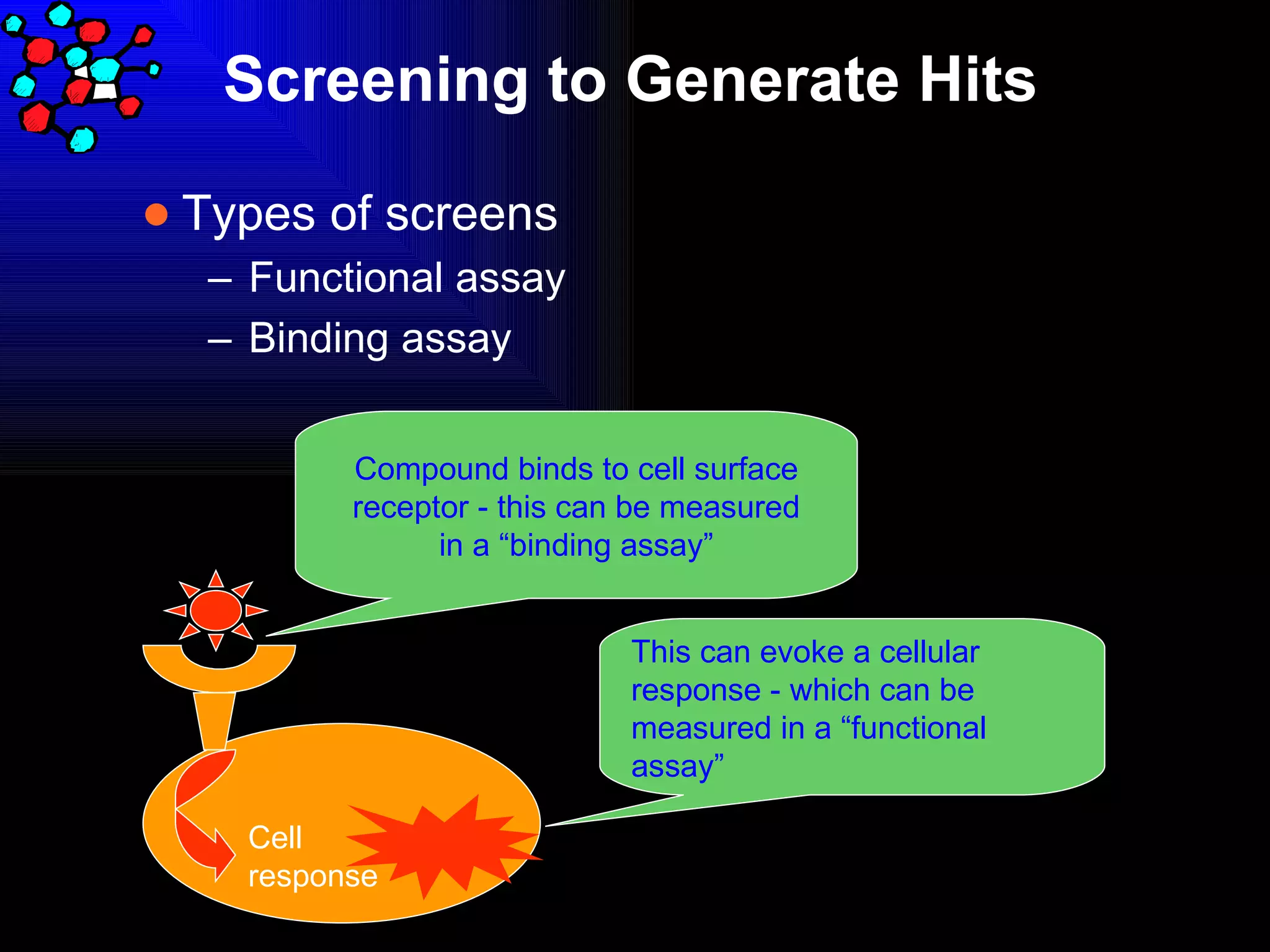
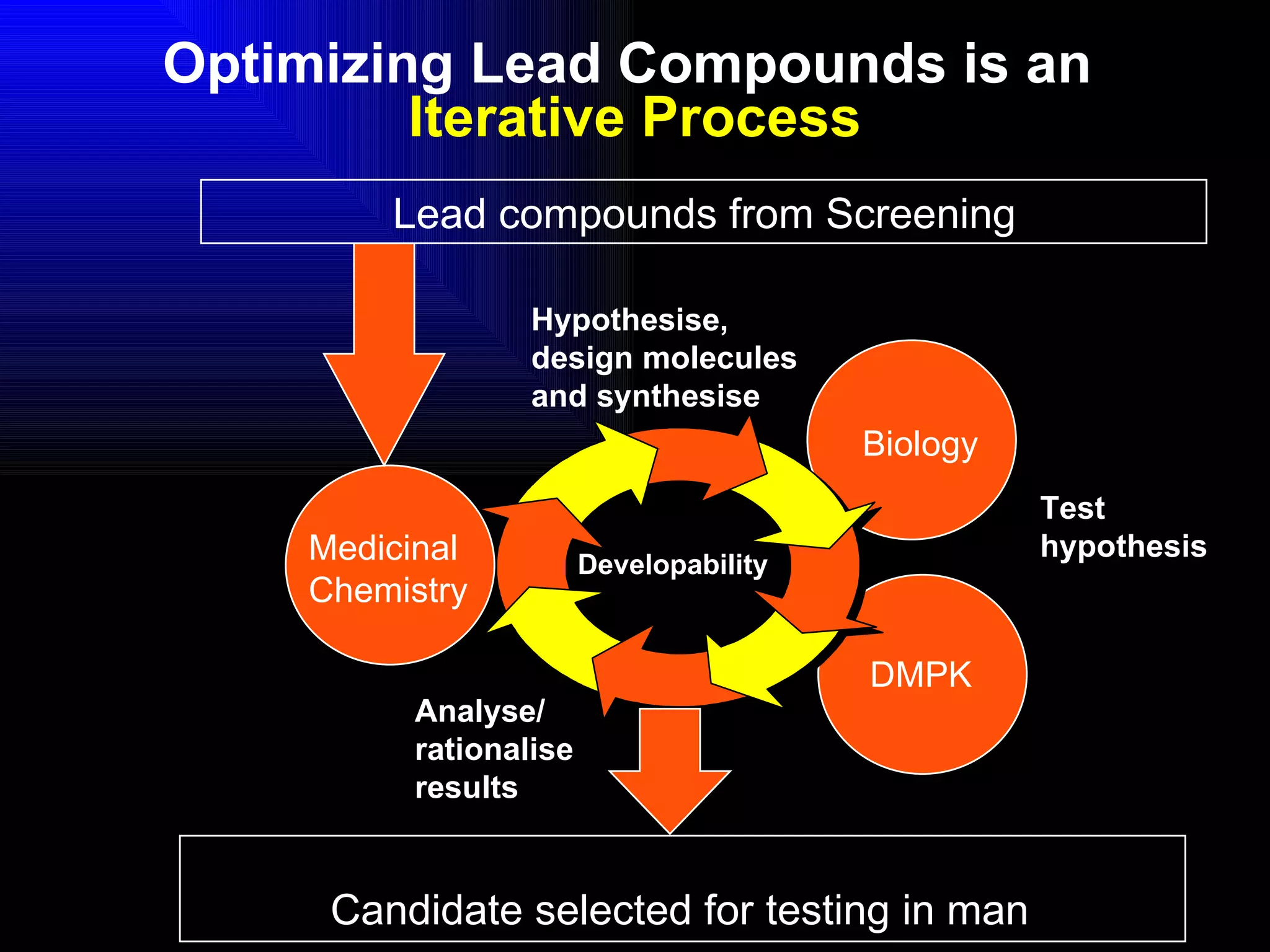
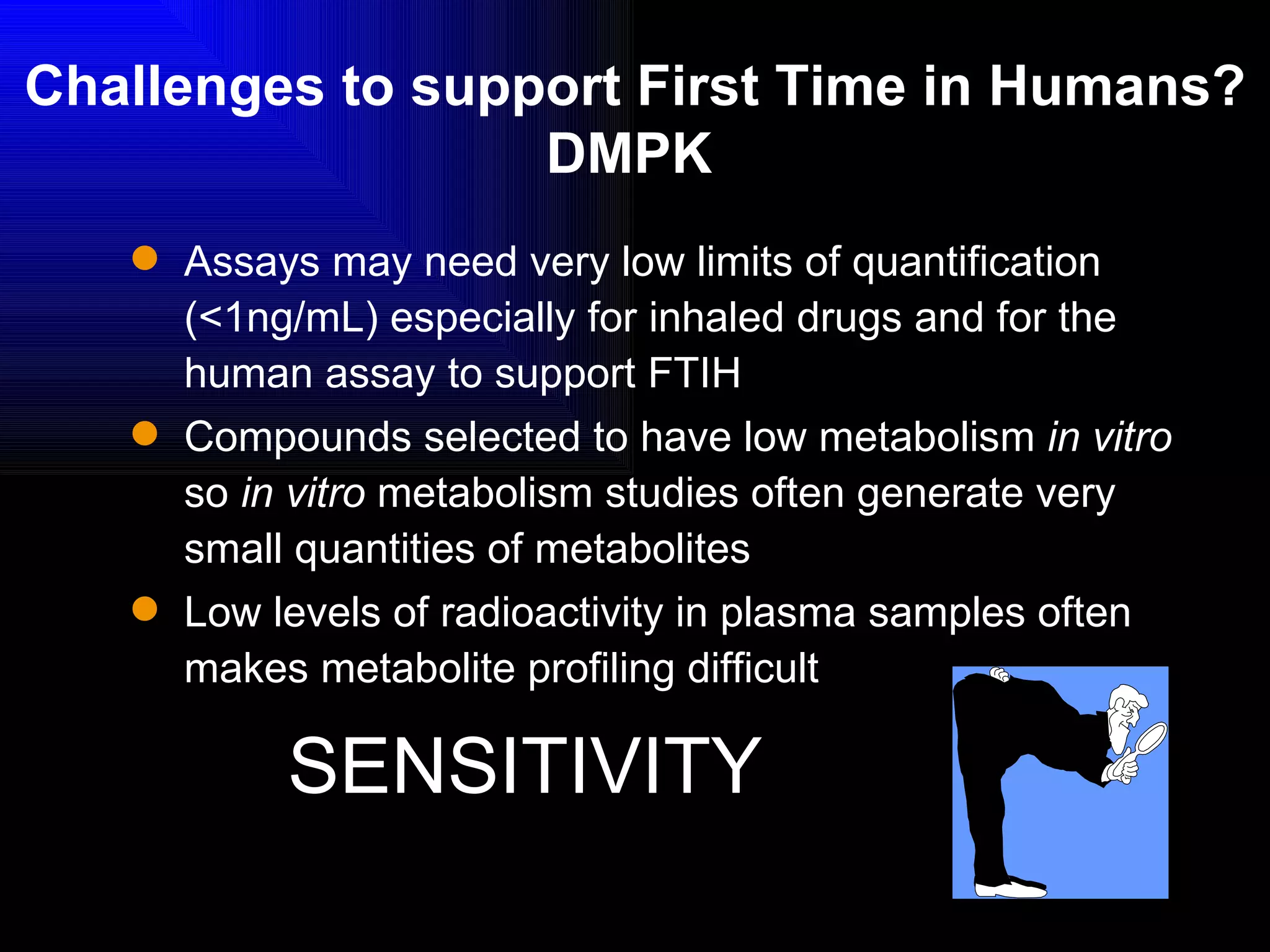
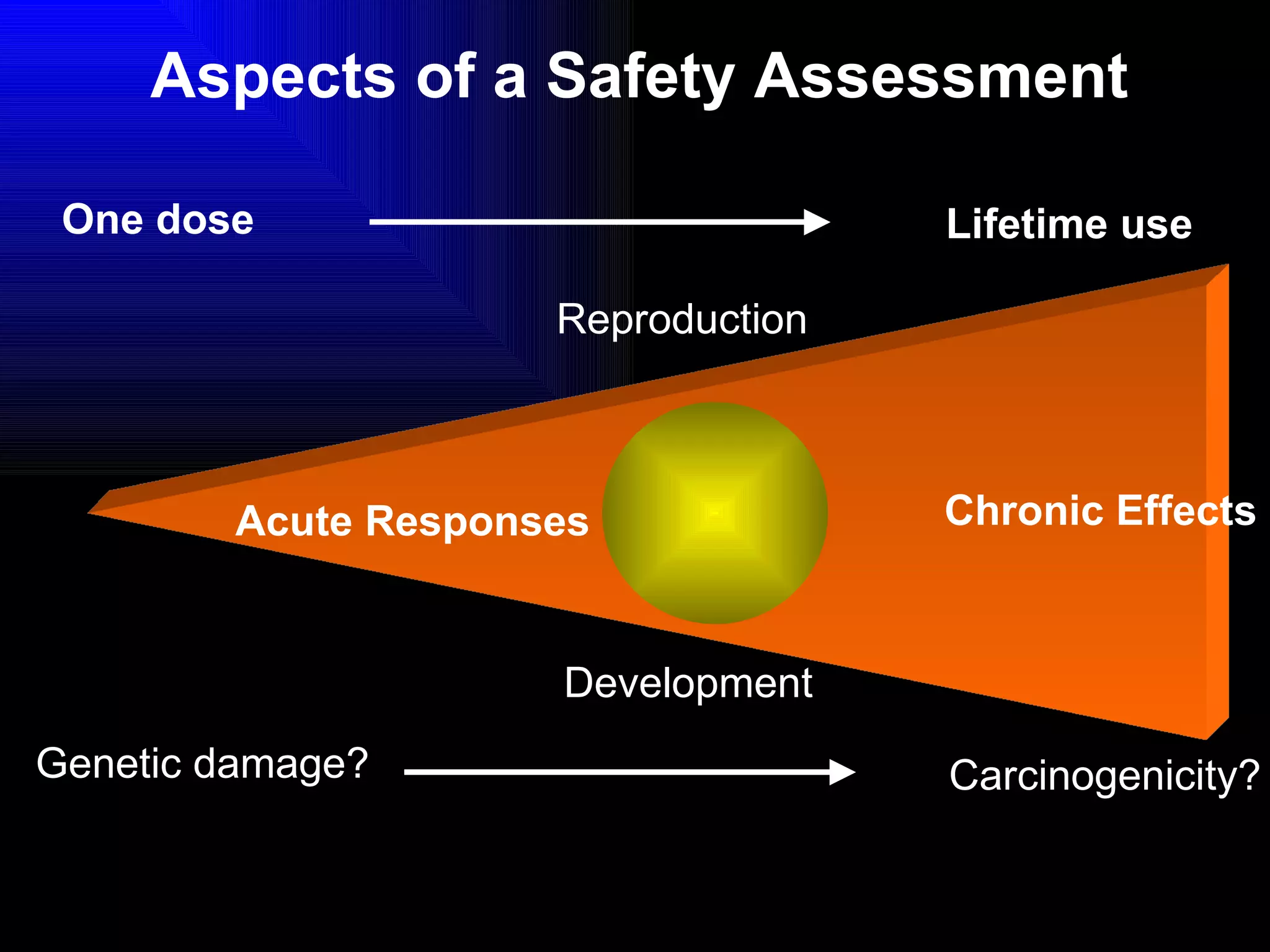
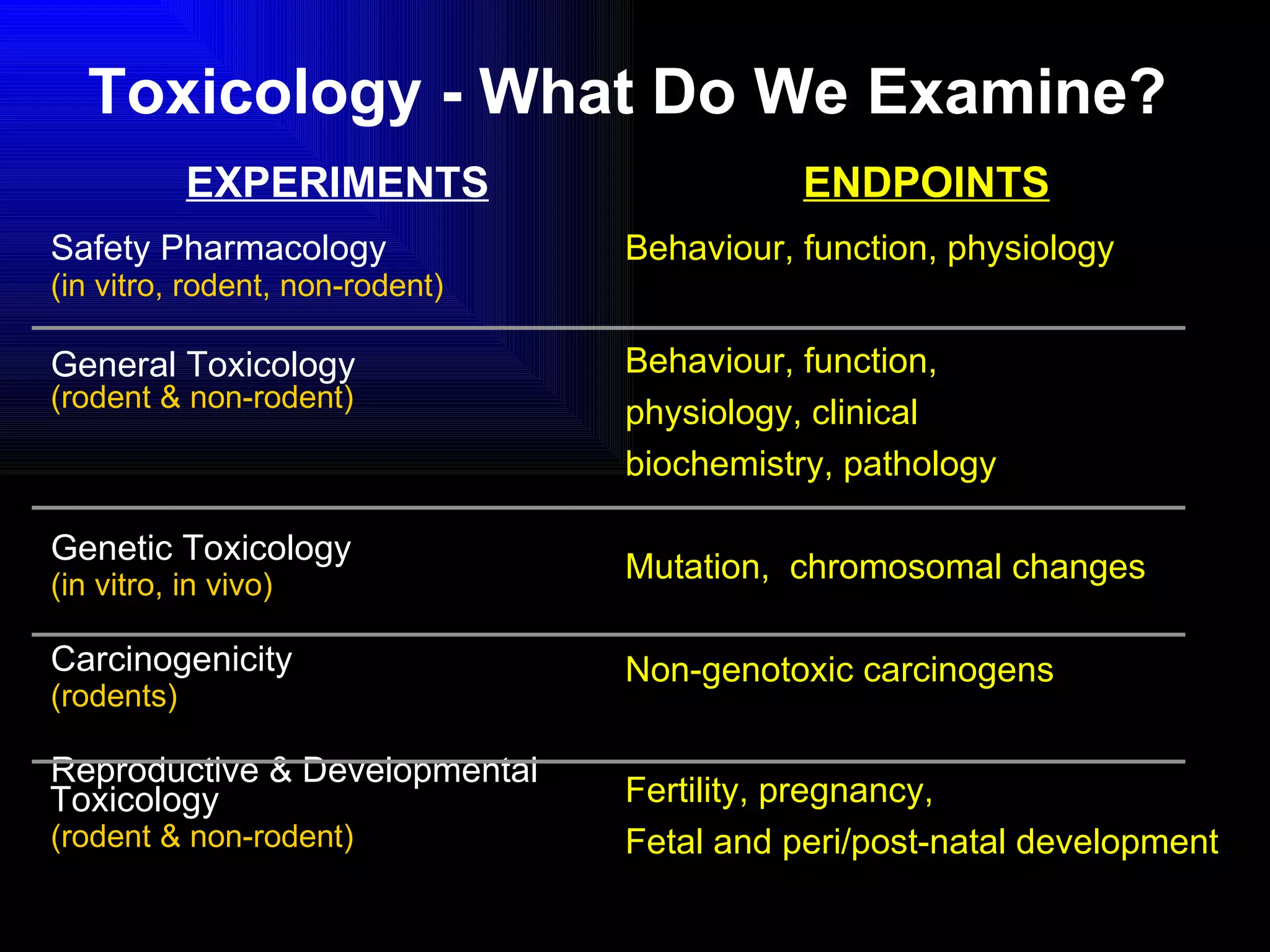
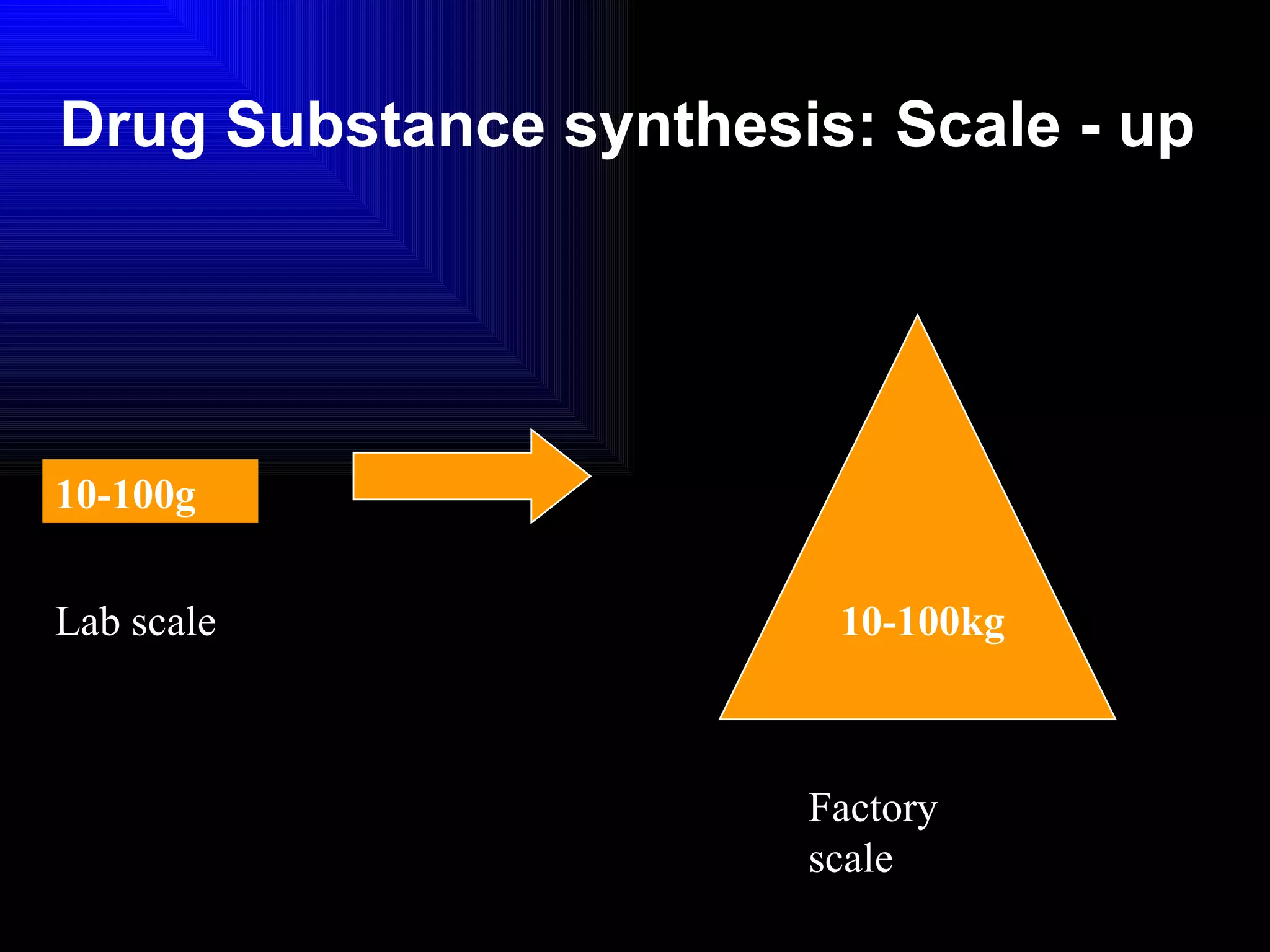
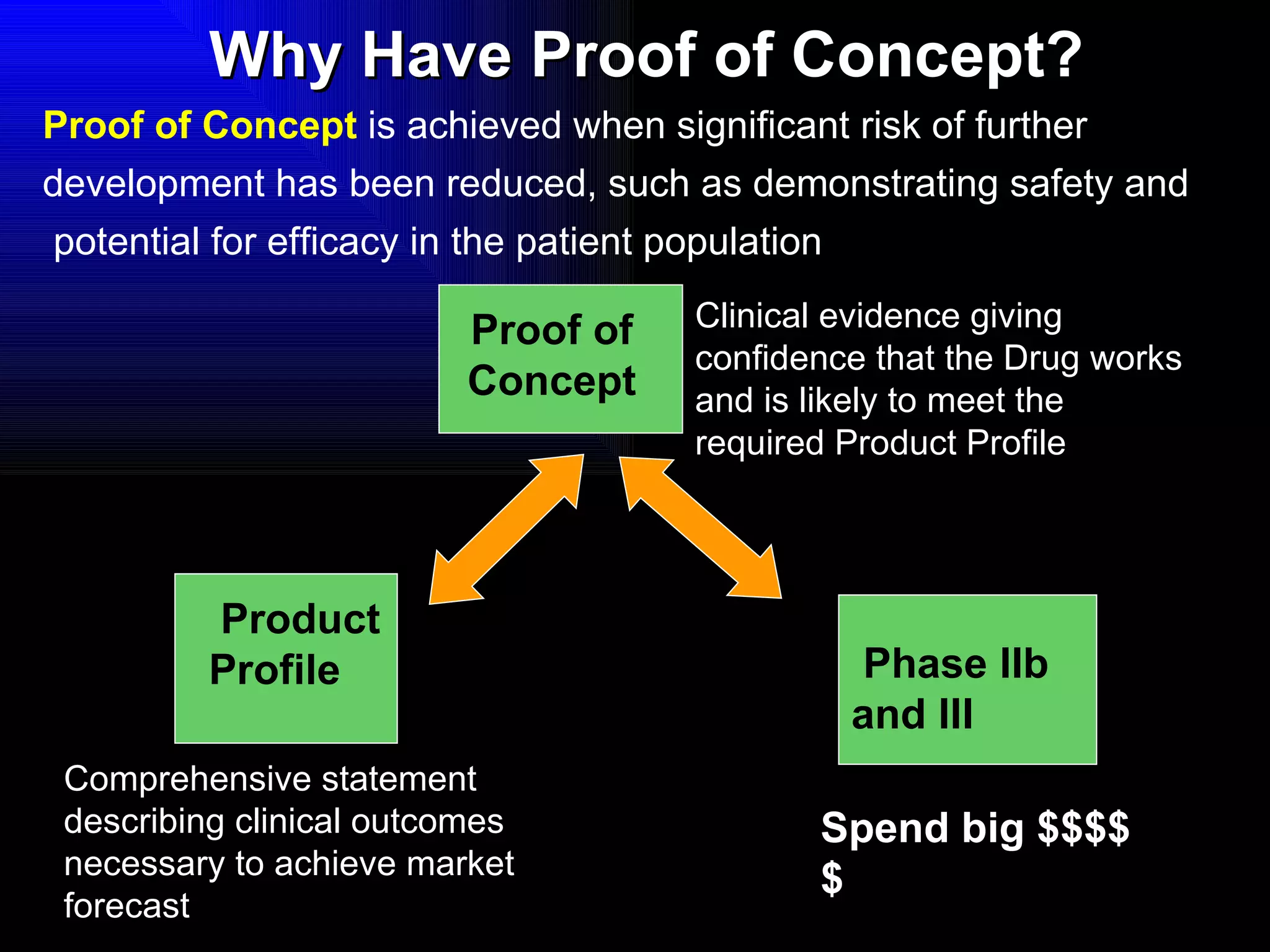
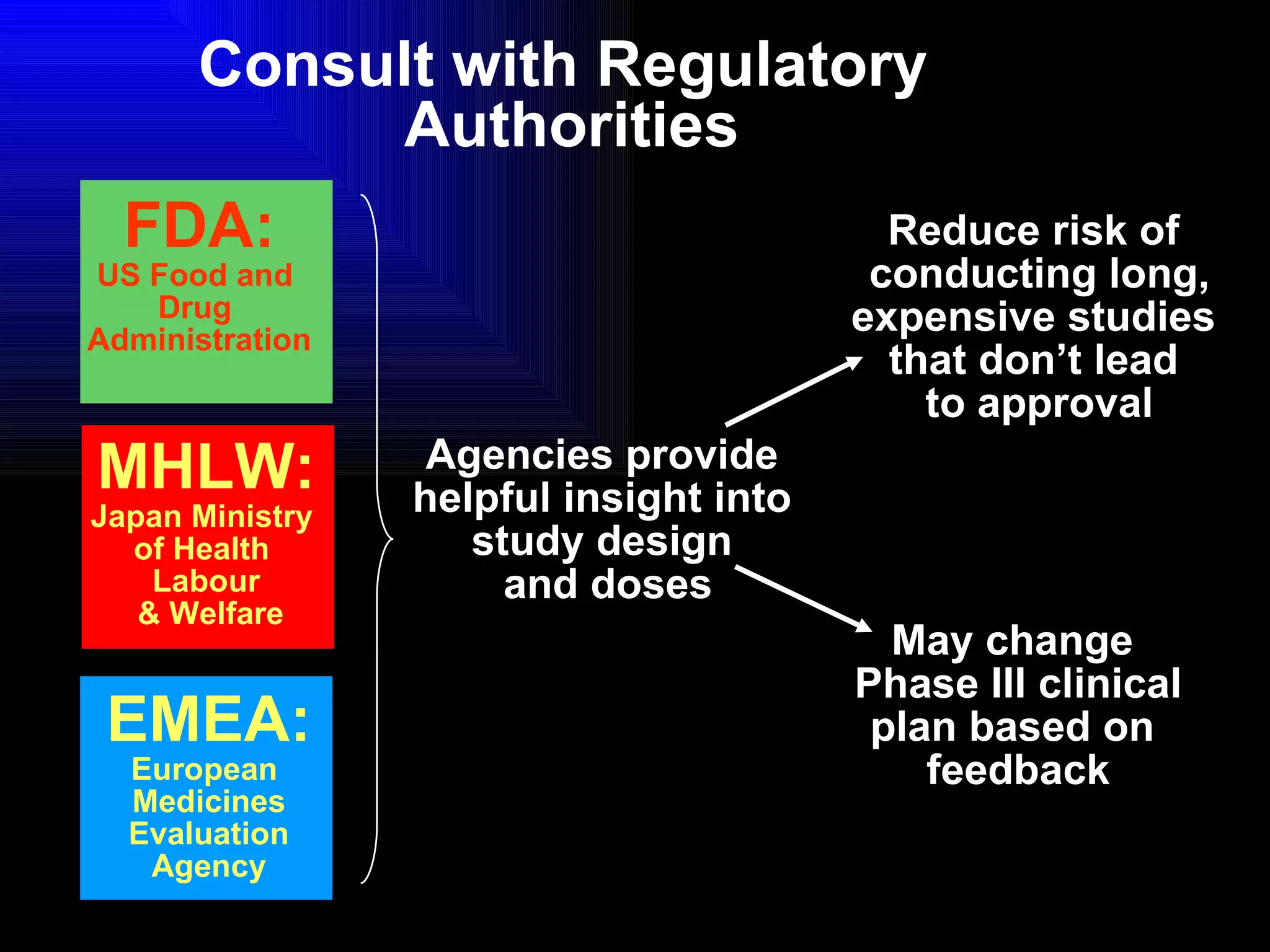
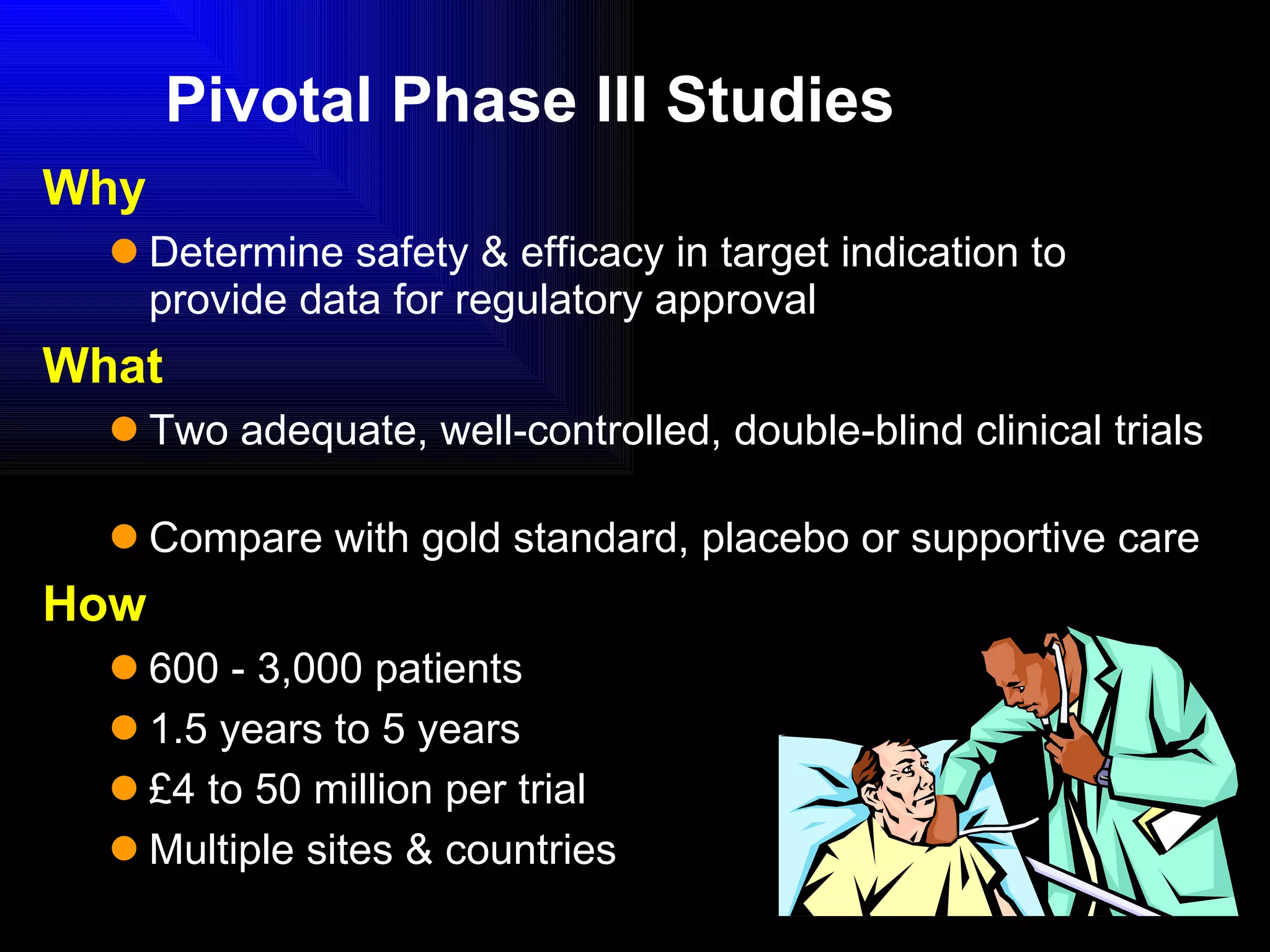
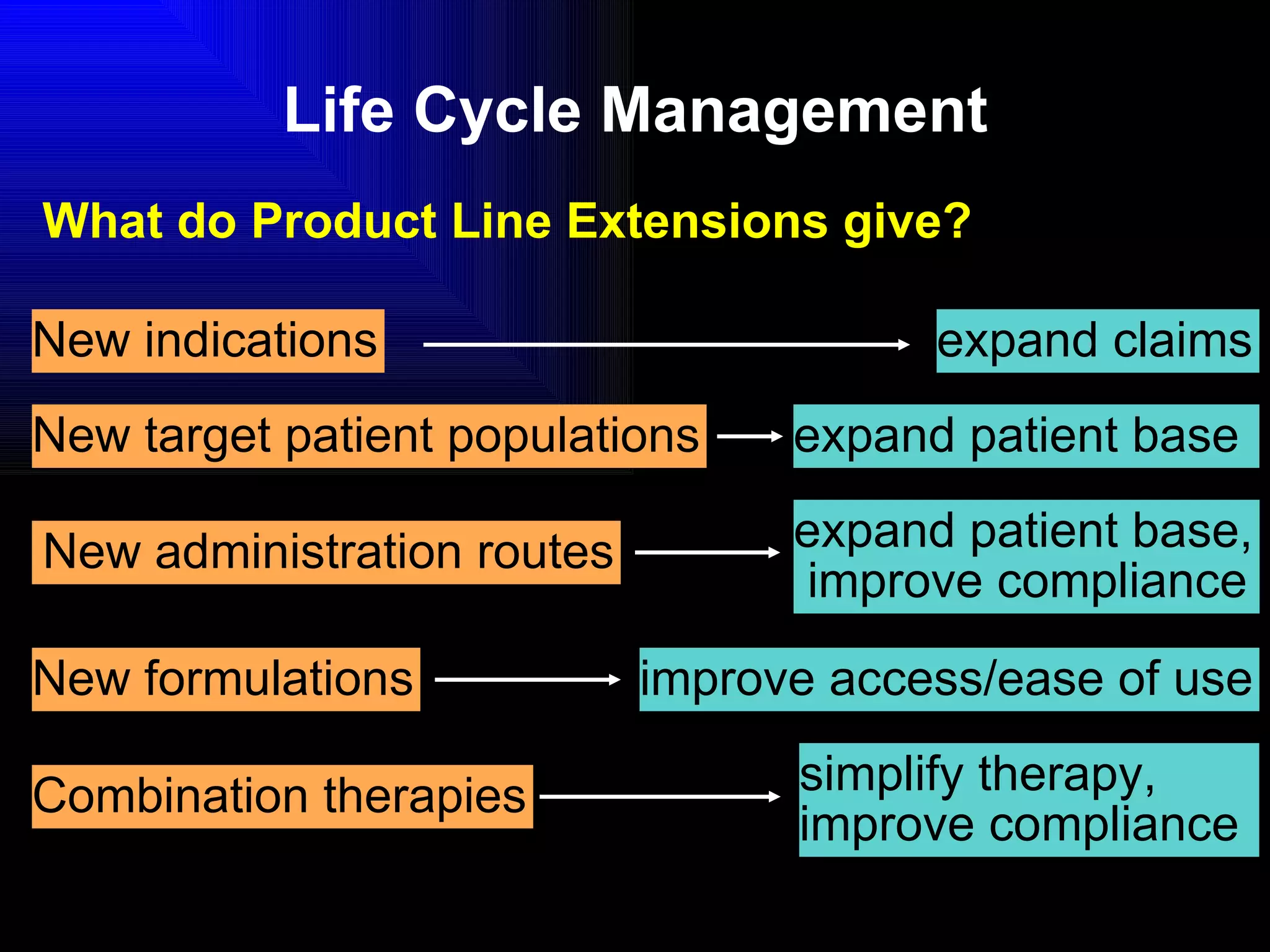
The document discusses the key stages in the drug discovery and development process including target selection, compound screening and hit optimization, selecting a drug candidate through further optimization of properties like absorption and metabolism, safety testing in animals and humans, proof of concept clinical trials in patients, large phase 3 clinical trials for registration and approval, and finally launch and life cycle management. It notes that the entire process from discovery to approval can take 12-16 years and cost over $1 billion.