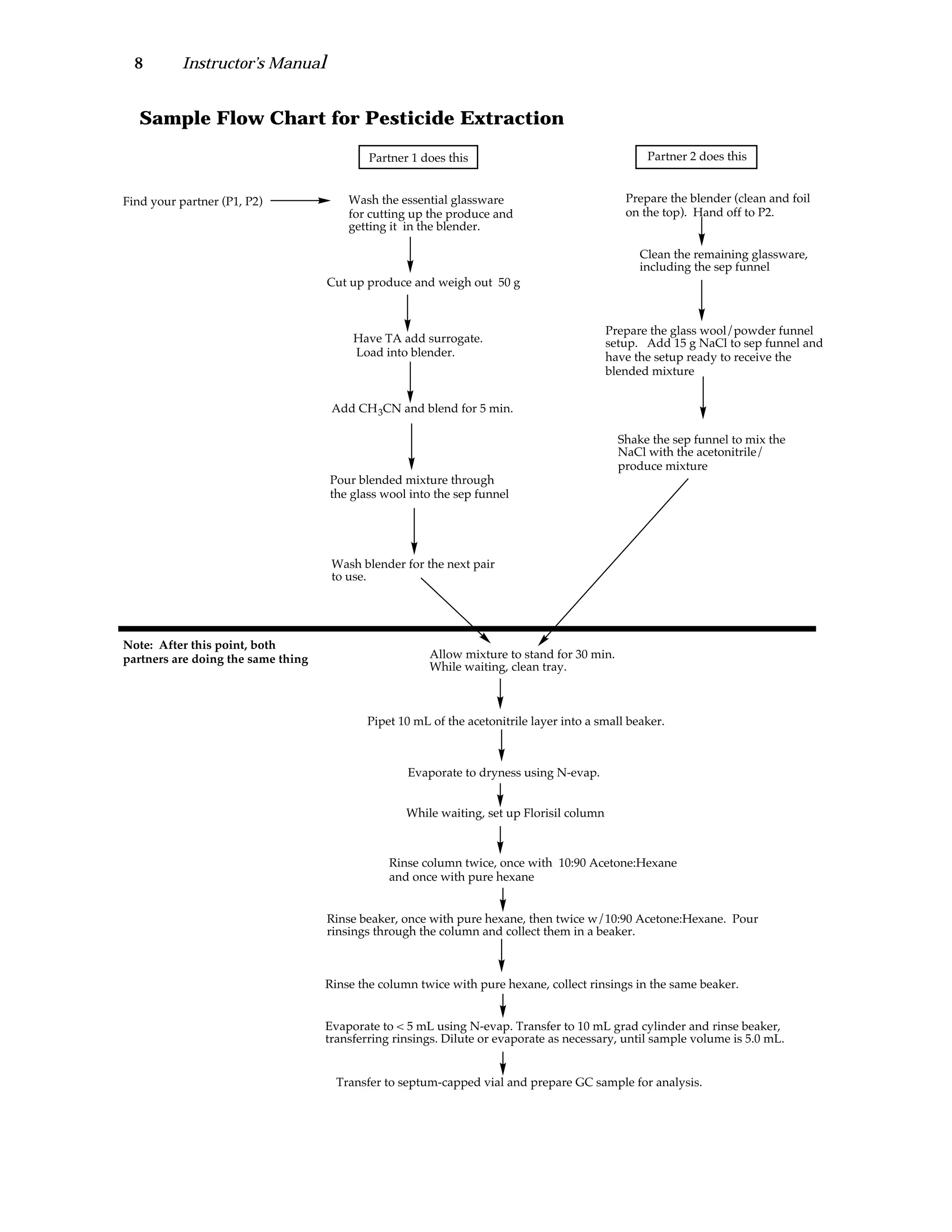
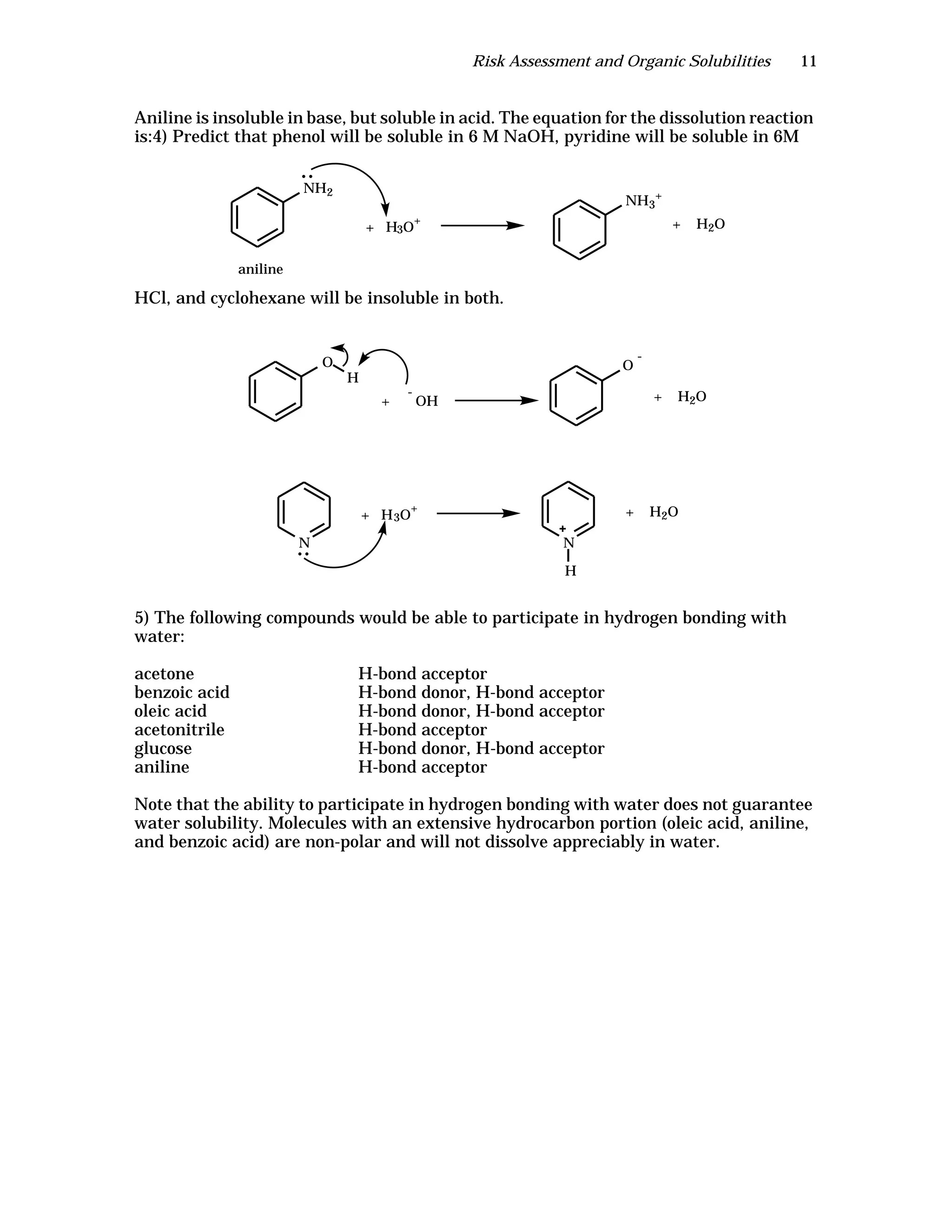
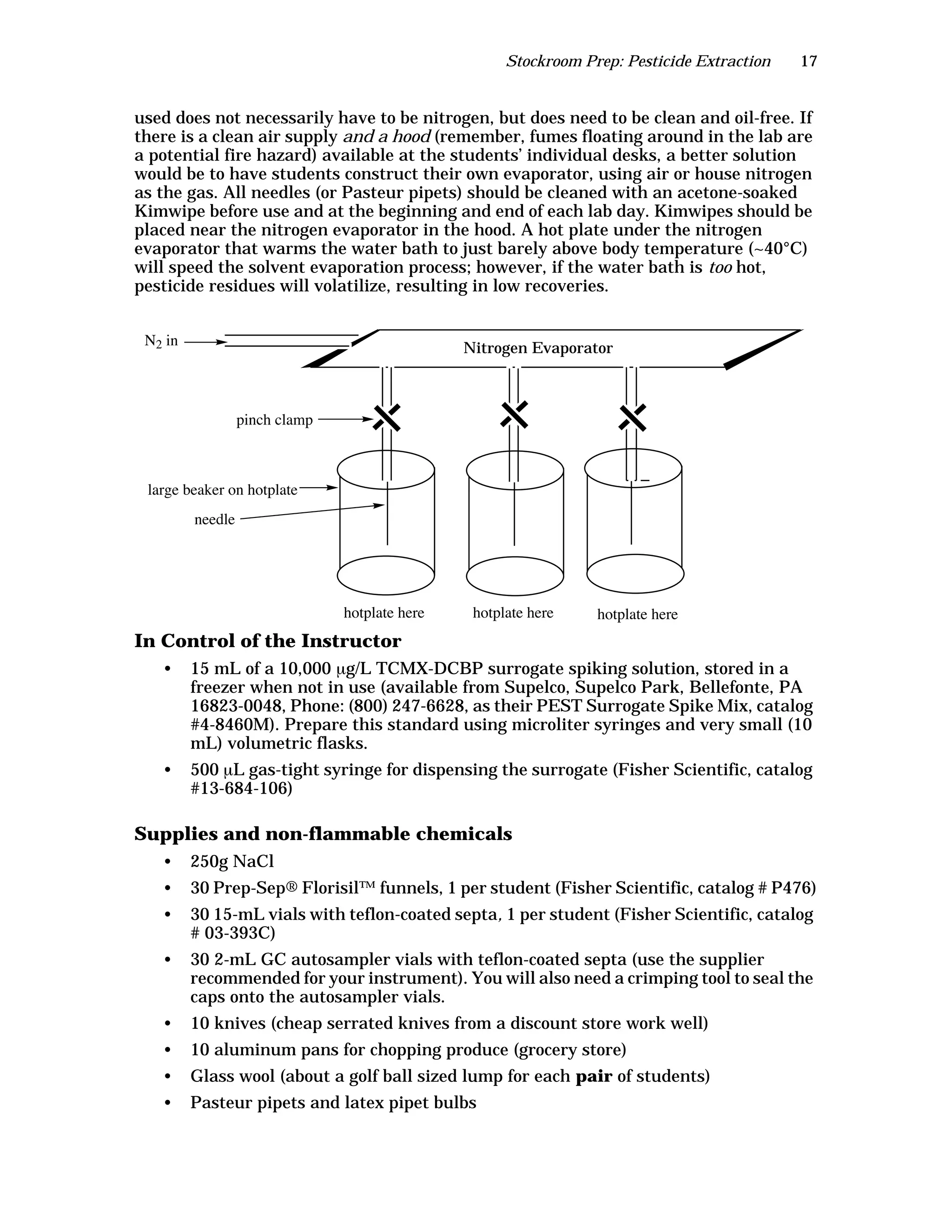
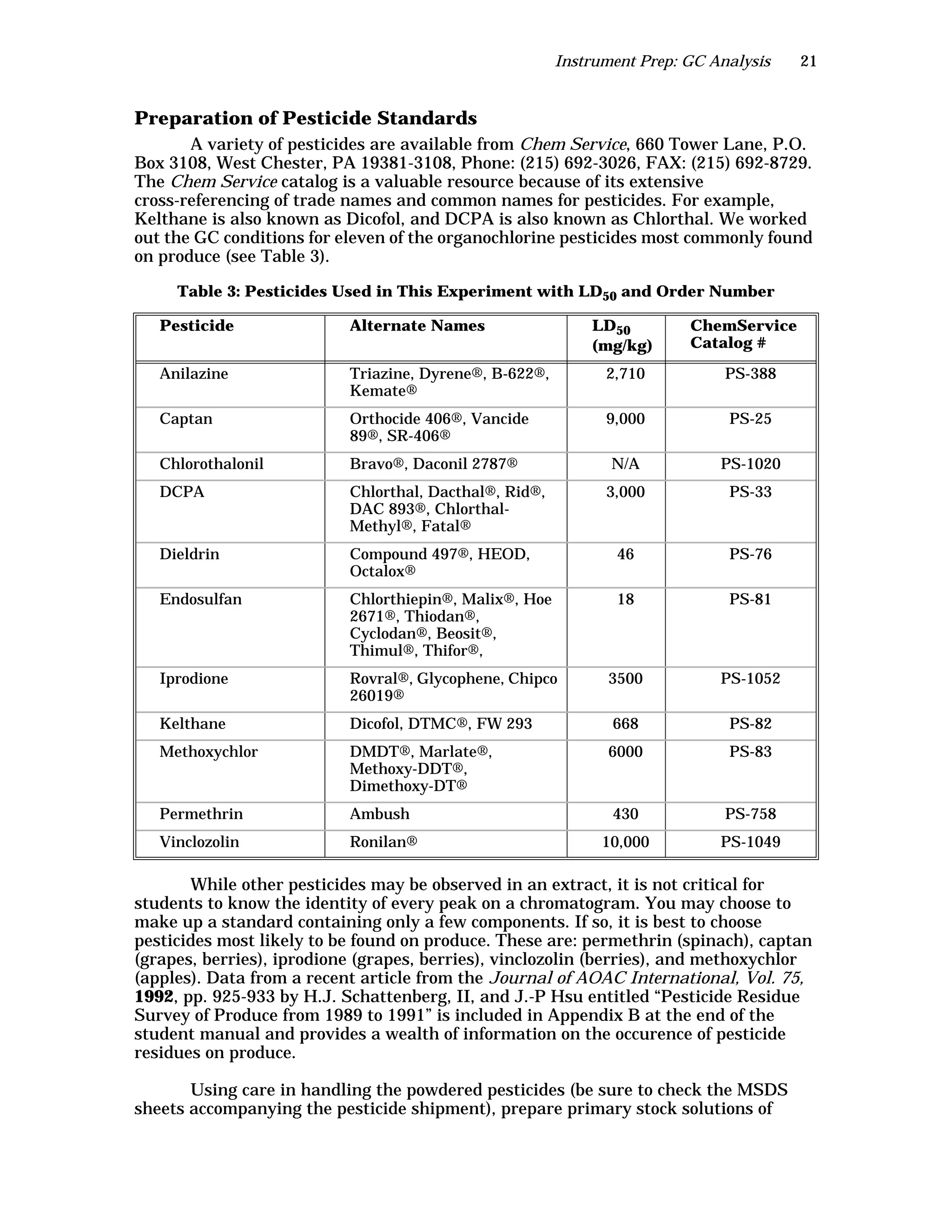
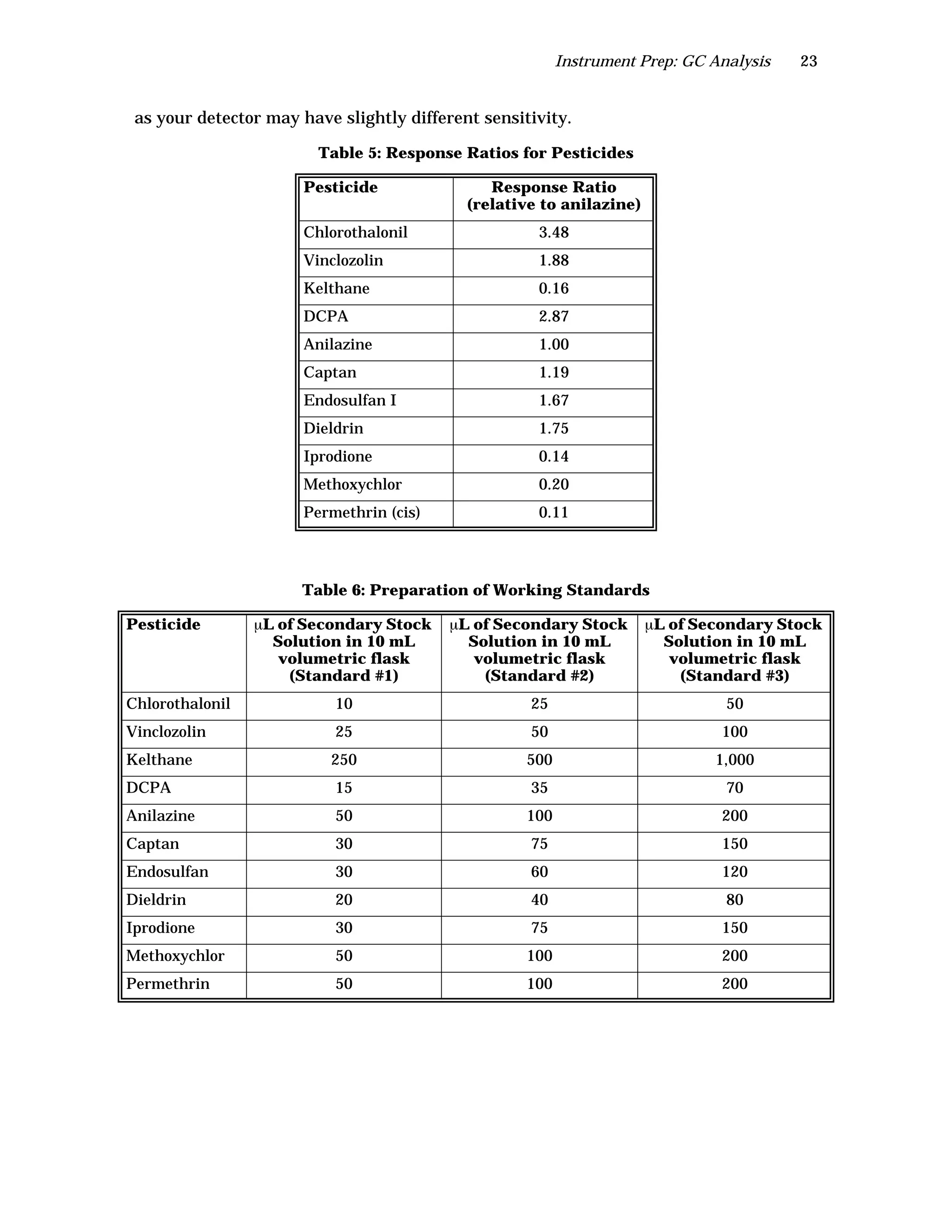
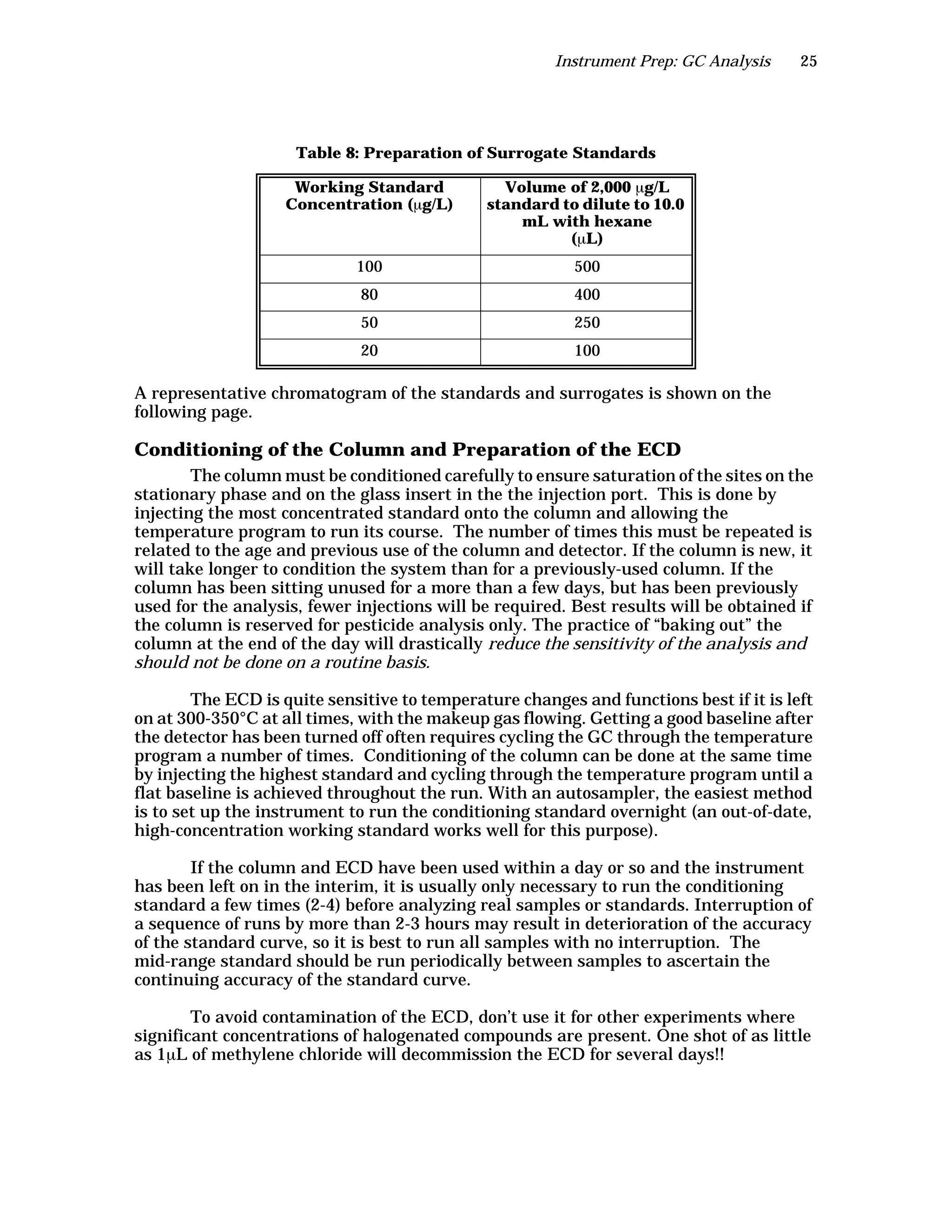
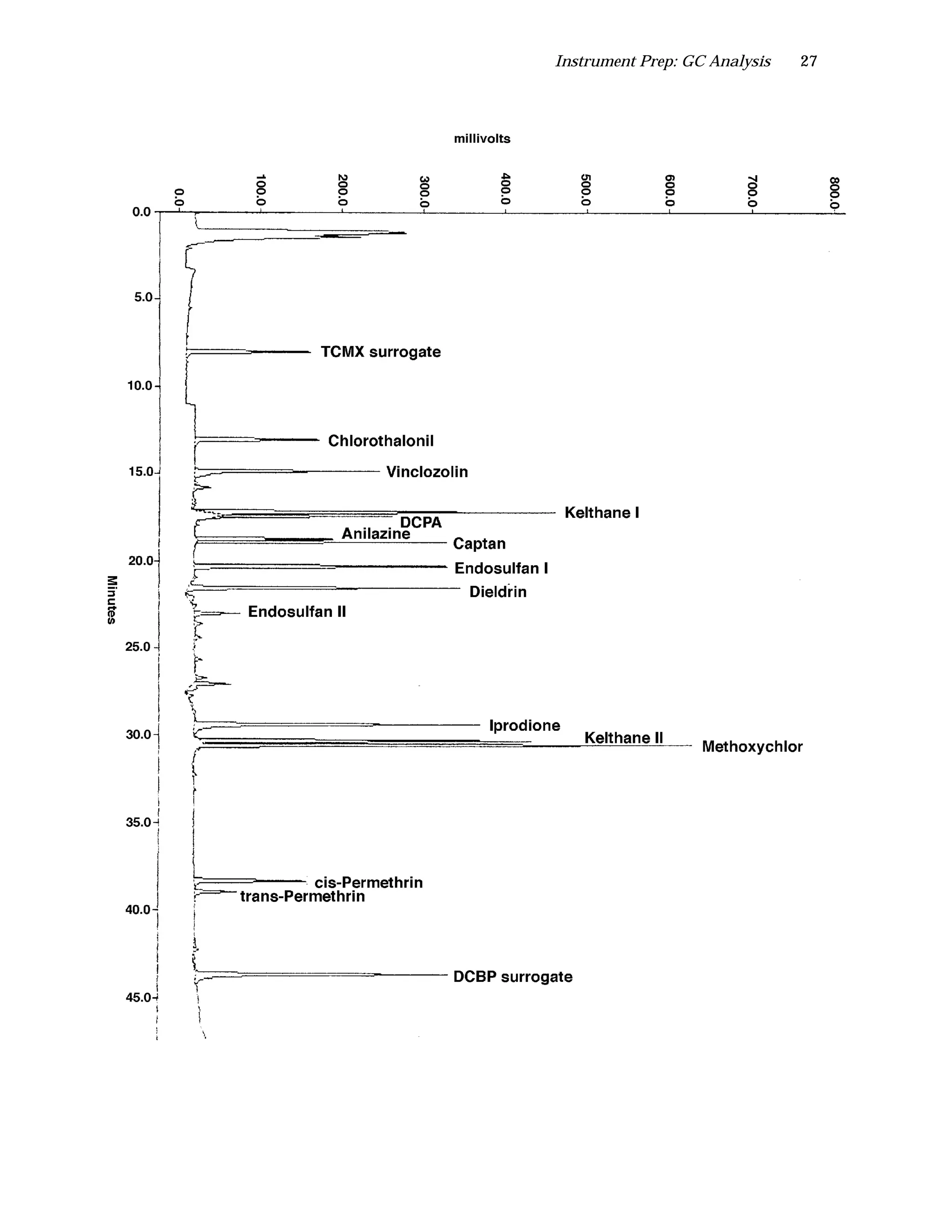
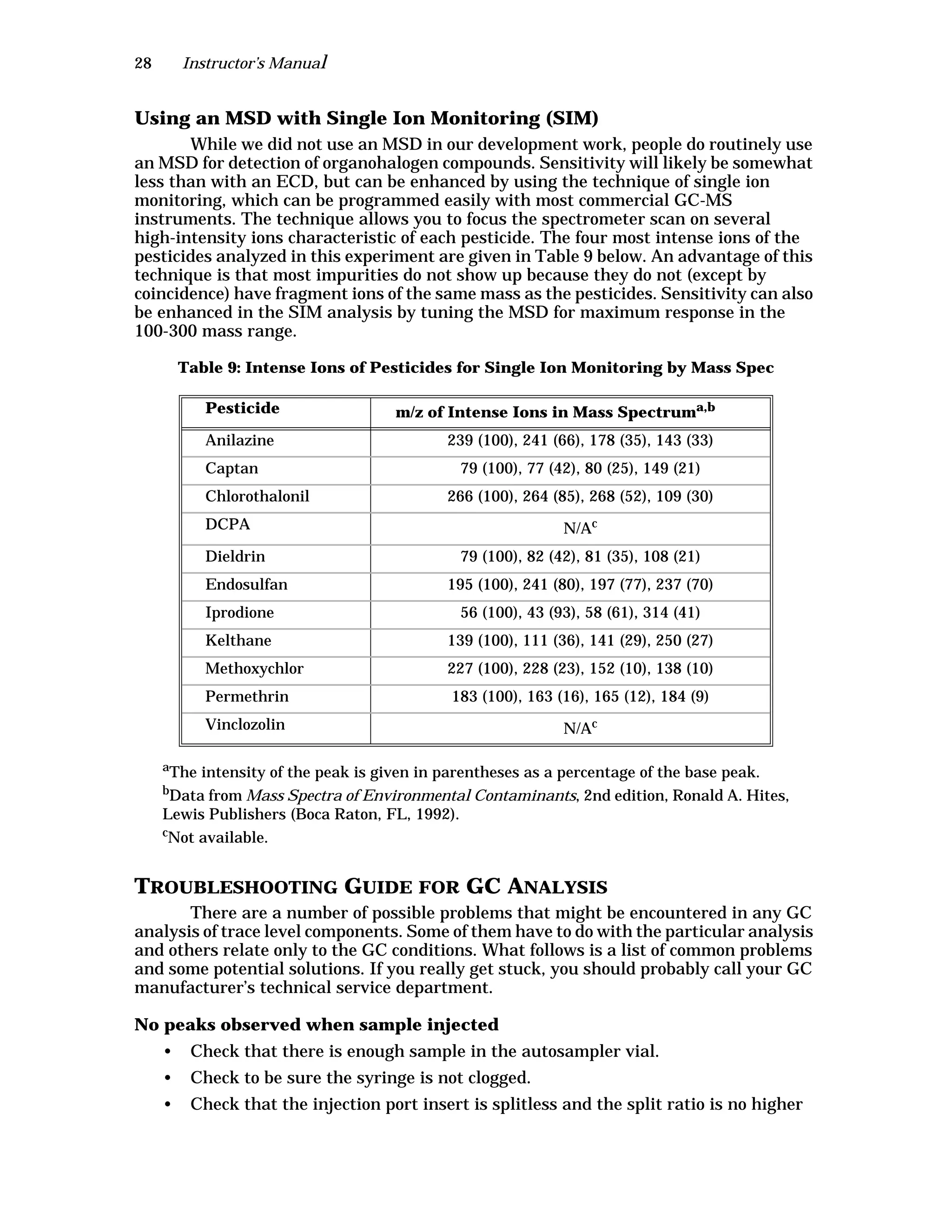
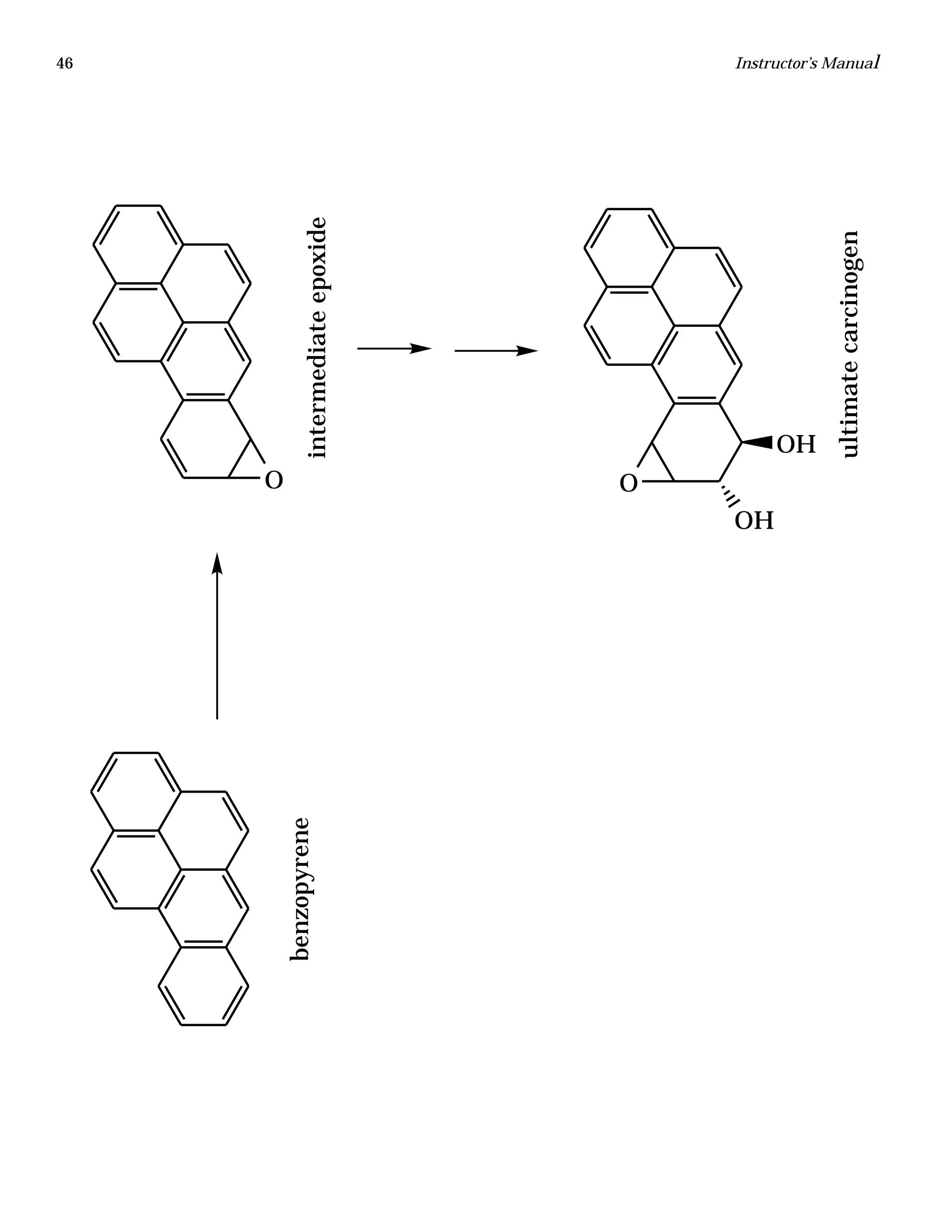
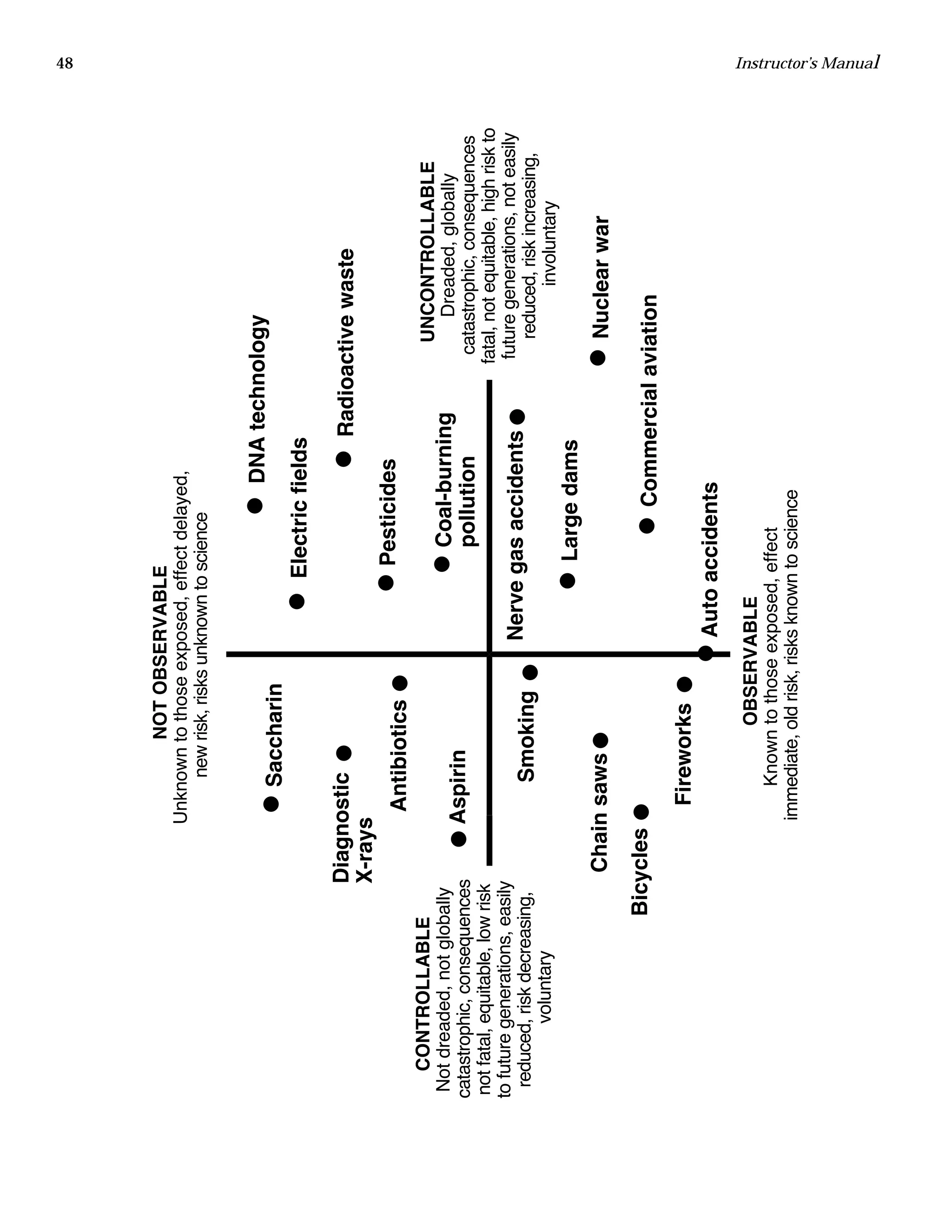
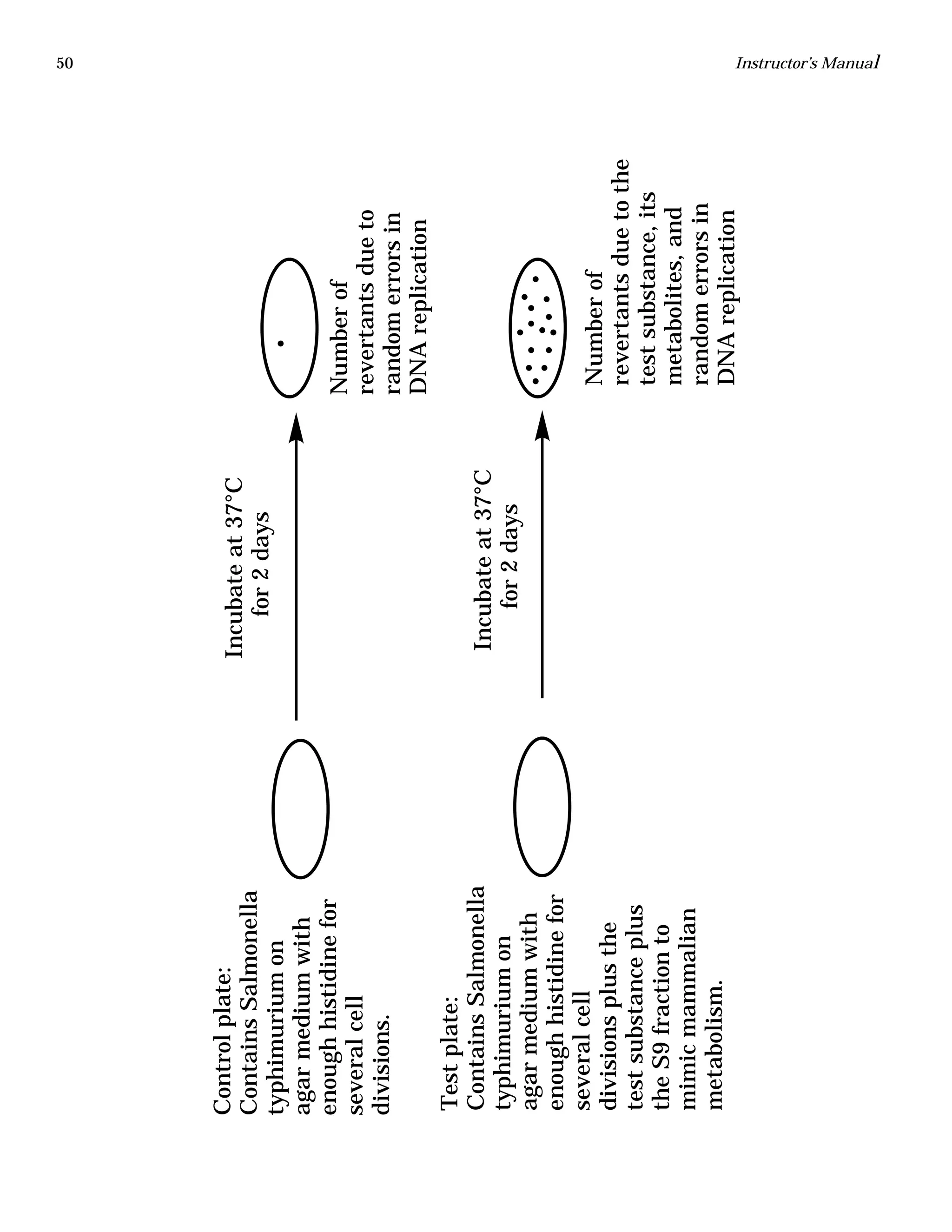
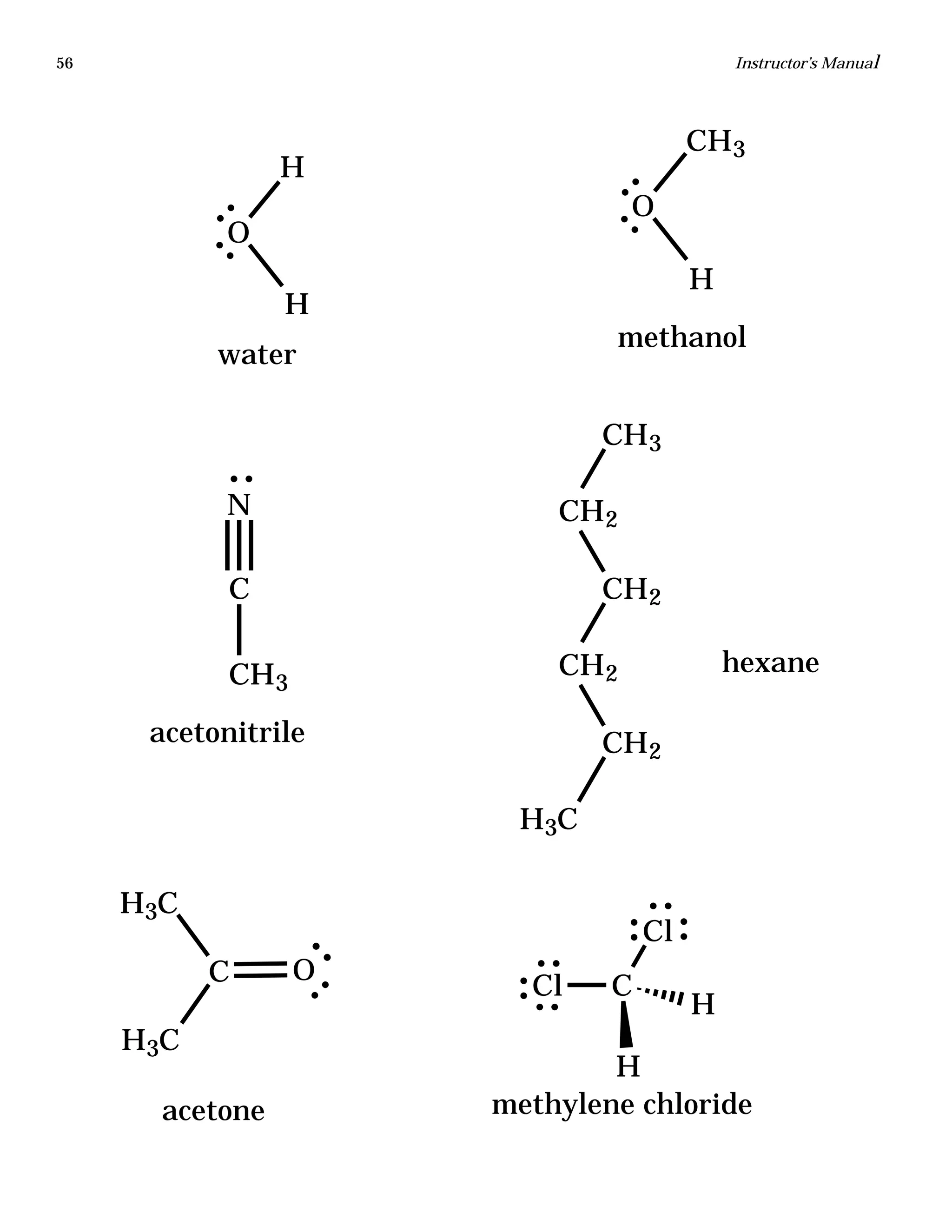
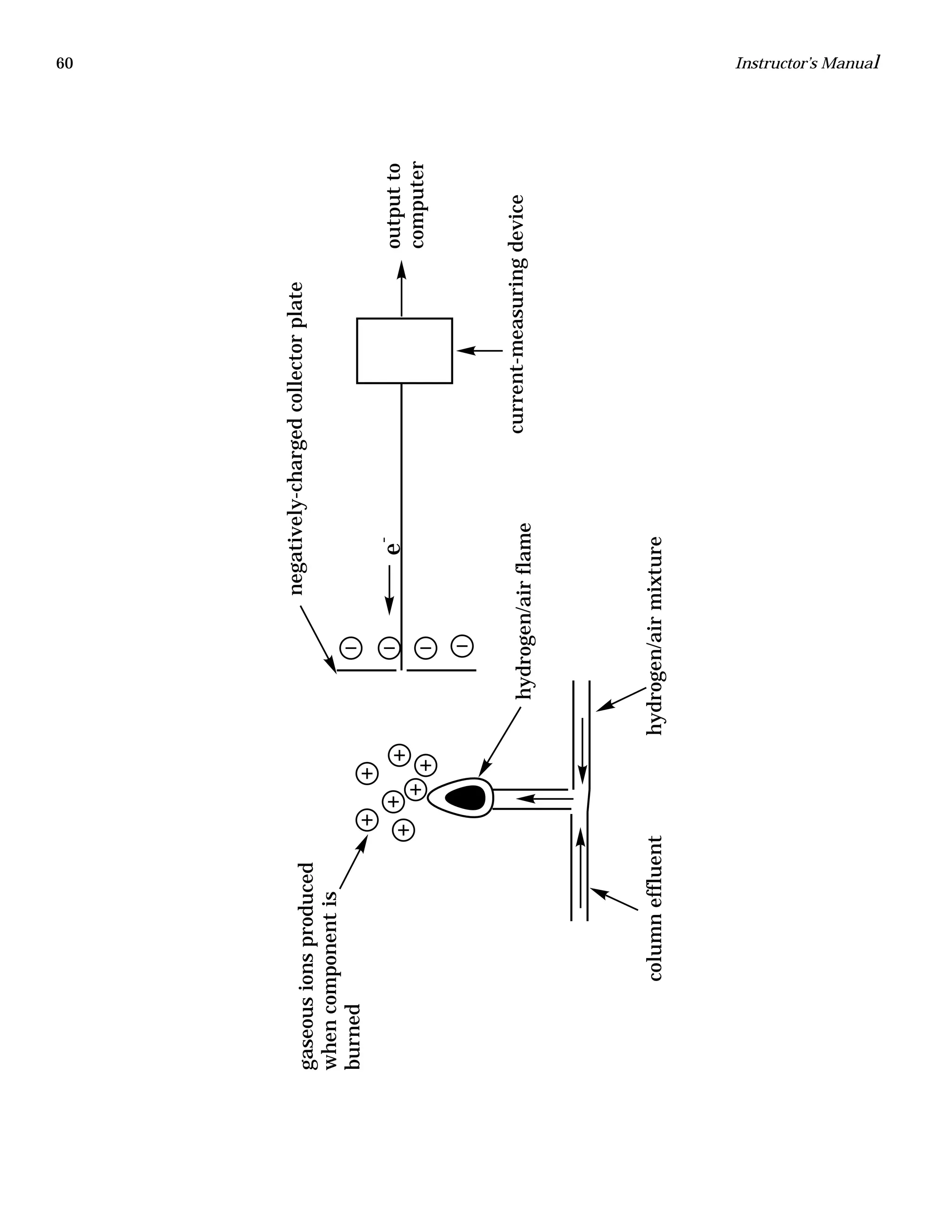
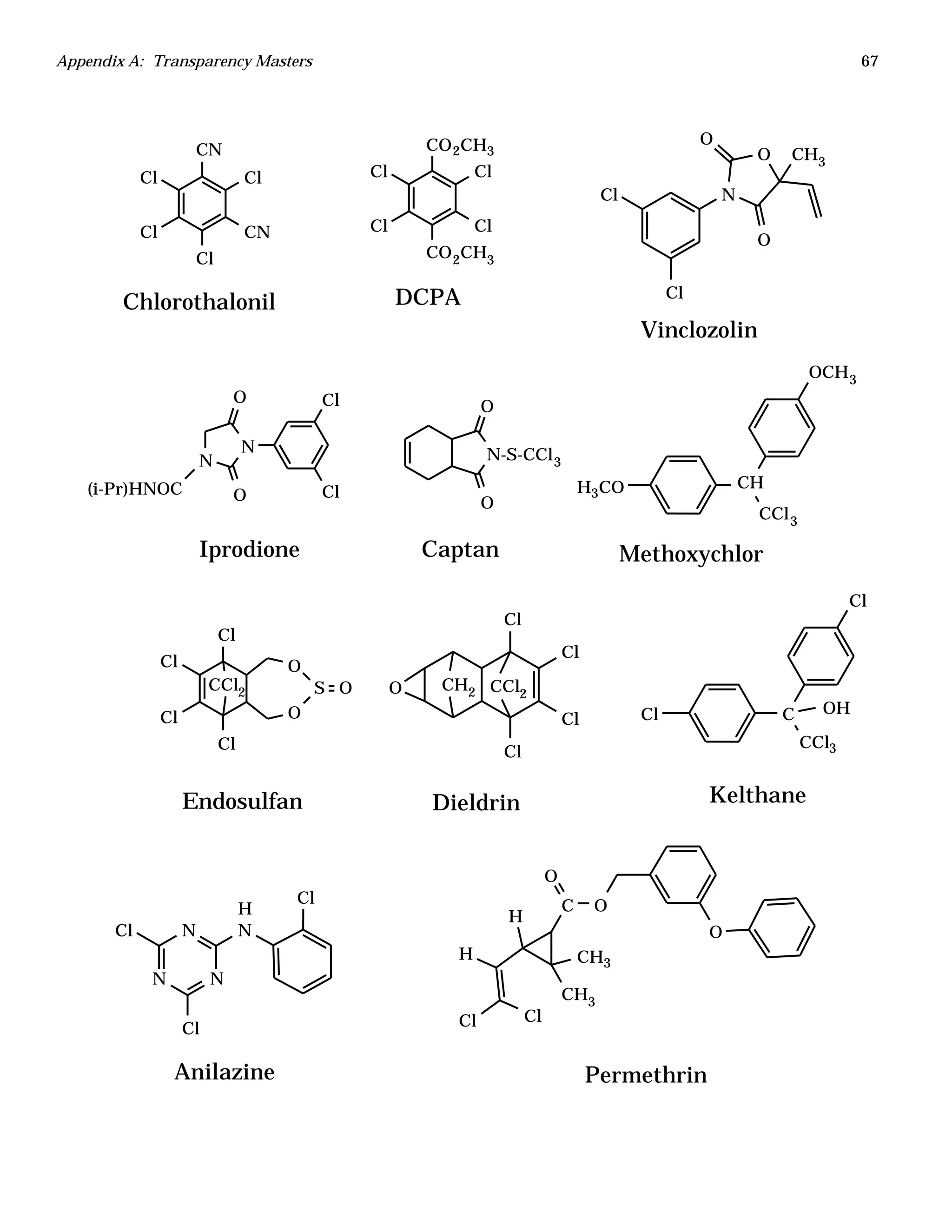
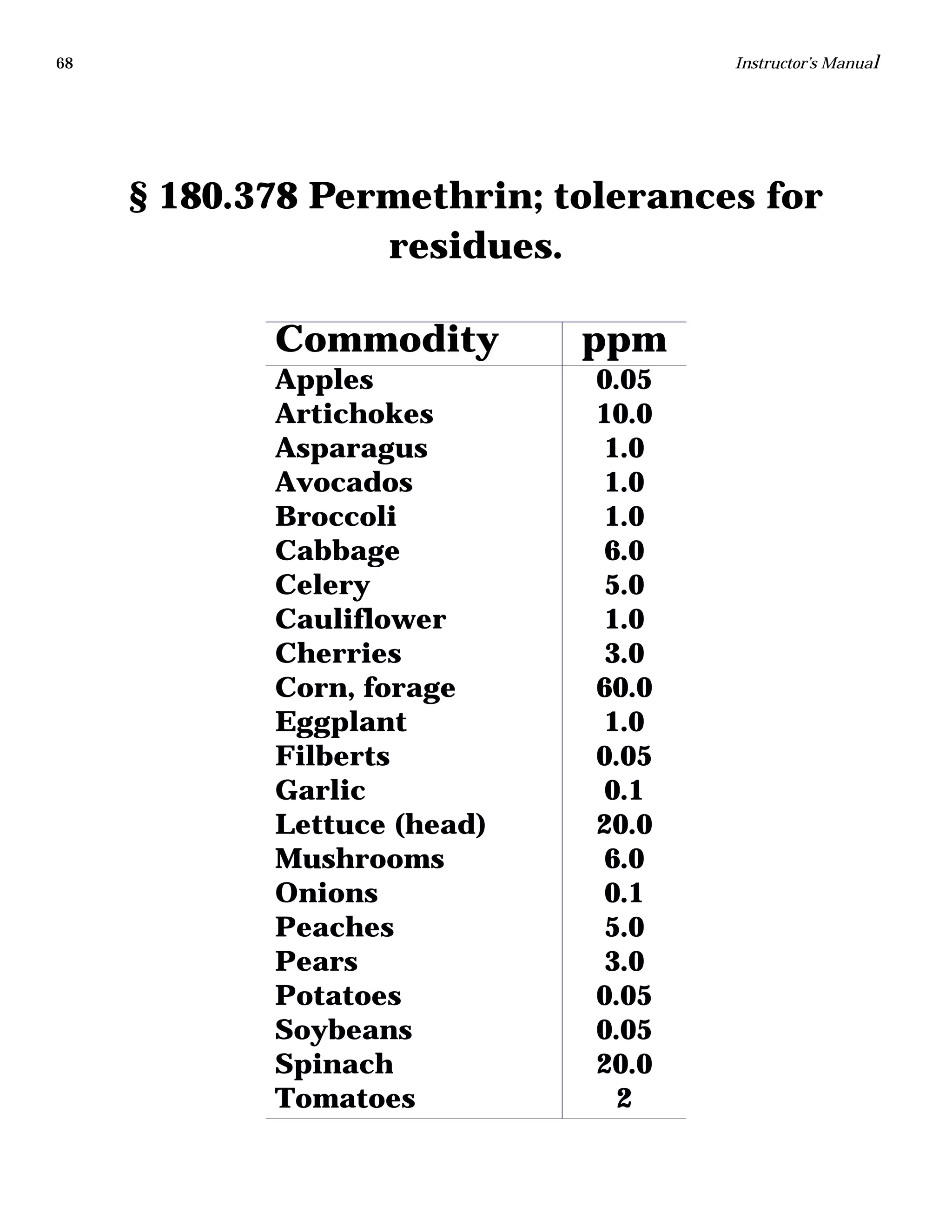
The document is an instructor's manual for a module on organochlorine pesticides in fruits and vegetables, detailing objectives, logistics, and procedures for teaching students about pesticide analysis through various experimental techniques such as gas chromatography. It outlines learning goals, risk assessment, extraction methods, and the debate over pesticides in the food supply, suitable for undergraduate chemistry courses. The manual encourages understanding of pesticide behavior, solubility, and the implications of their use in agriculture.