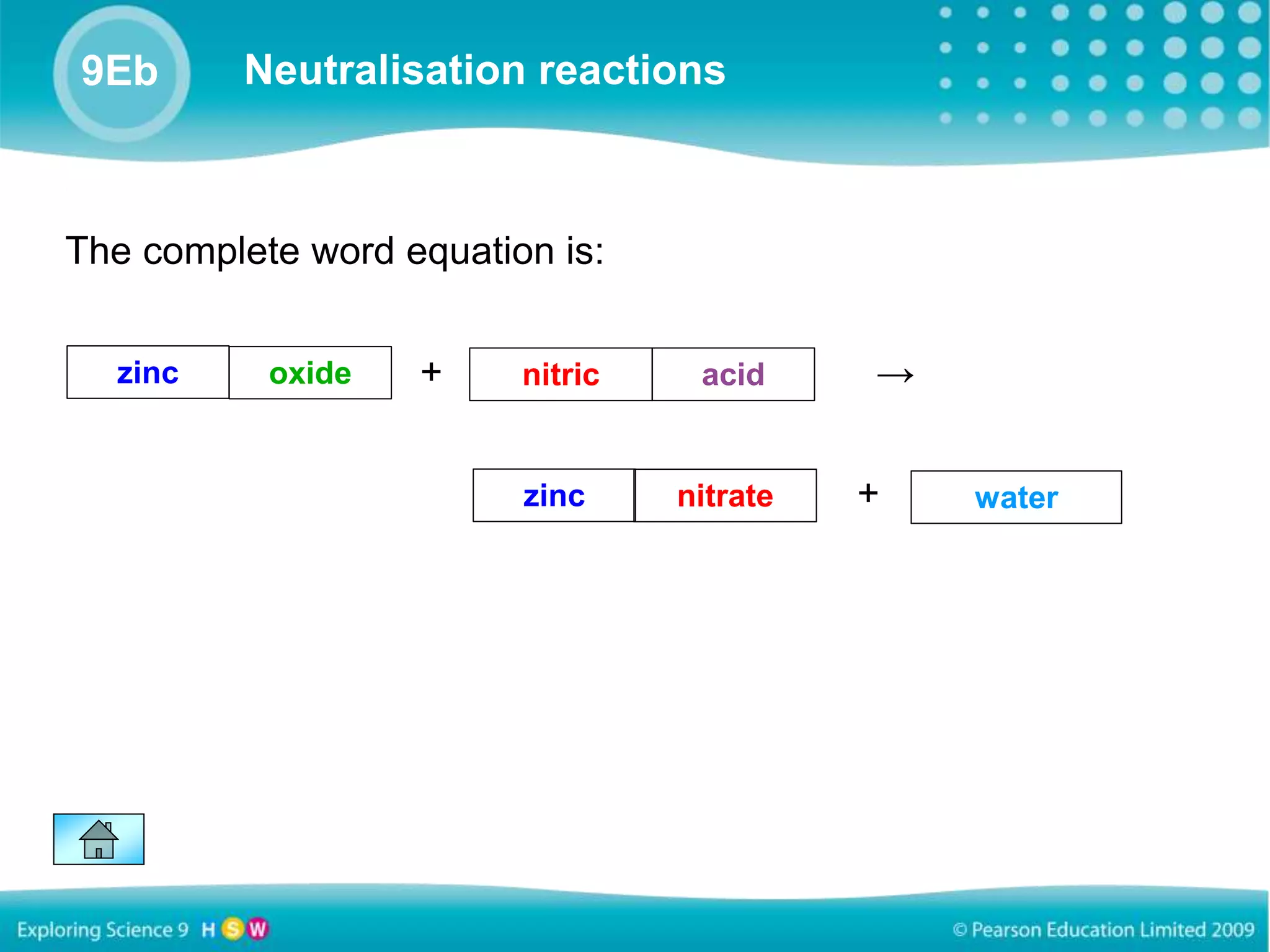
Neutralization reactions involve acids and bases reacting to form salts and water. The general word equation shows an acid and base yielding a salt and water. Models can represent these reactions through word equations showing the parts separating or symbol equations showing the chemical formulas. For example, in the reaction of zinc oxide and nitric acid, the parts separate and zinc and nitric acid combine to form zinc nitrate, while oxide and acid combine to form water.