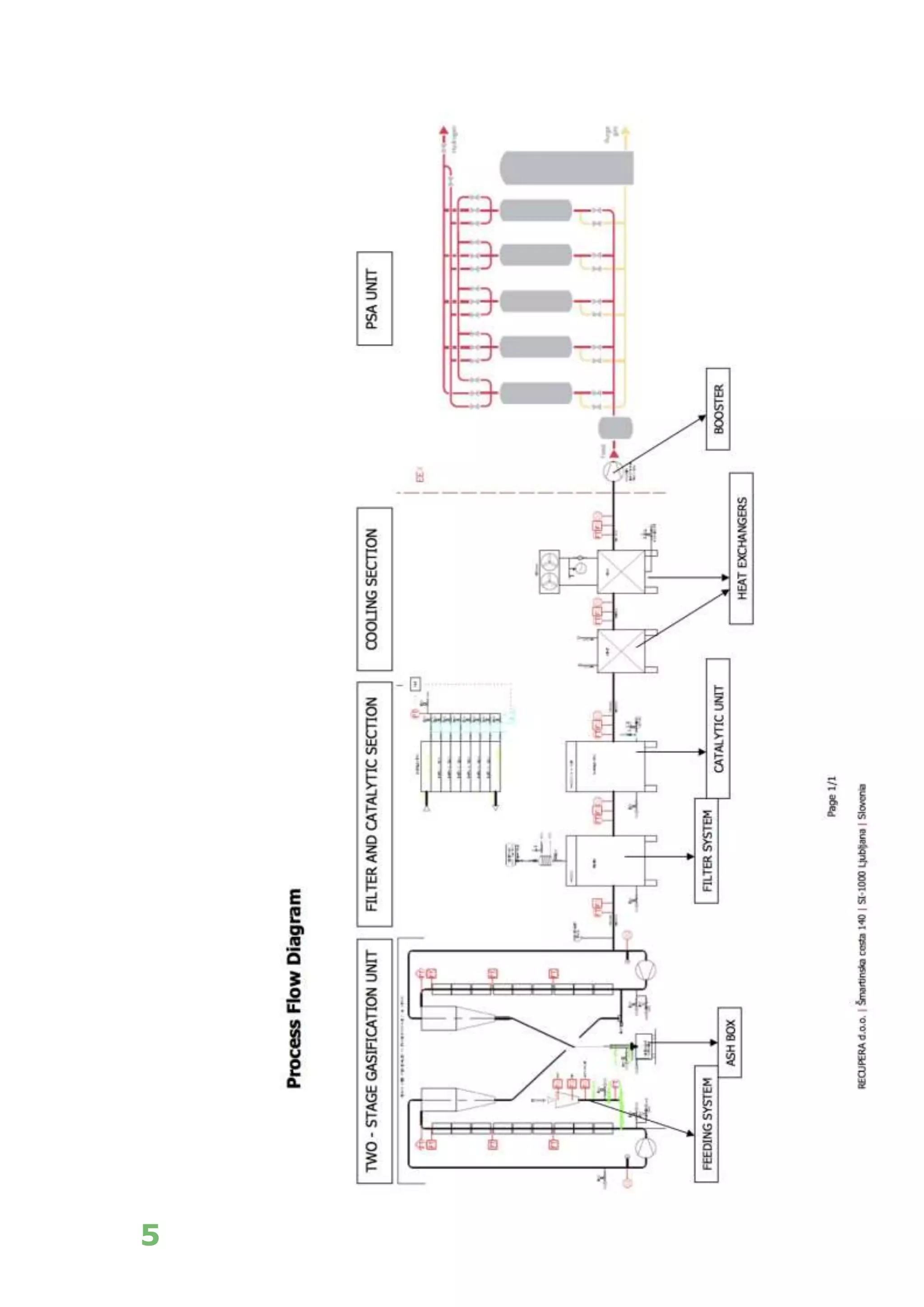
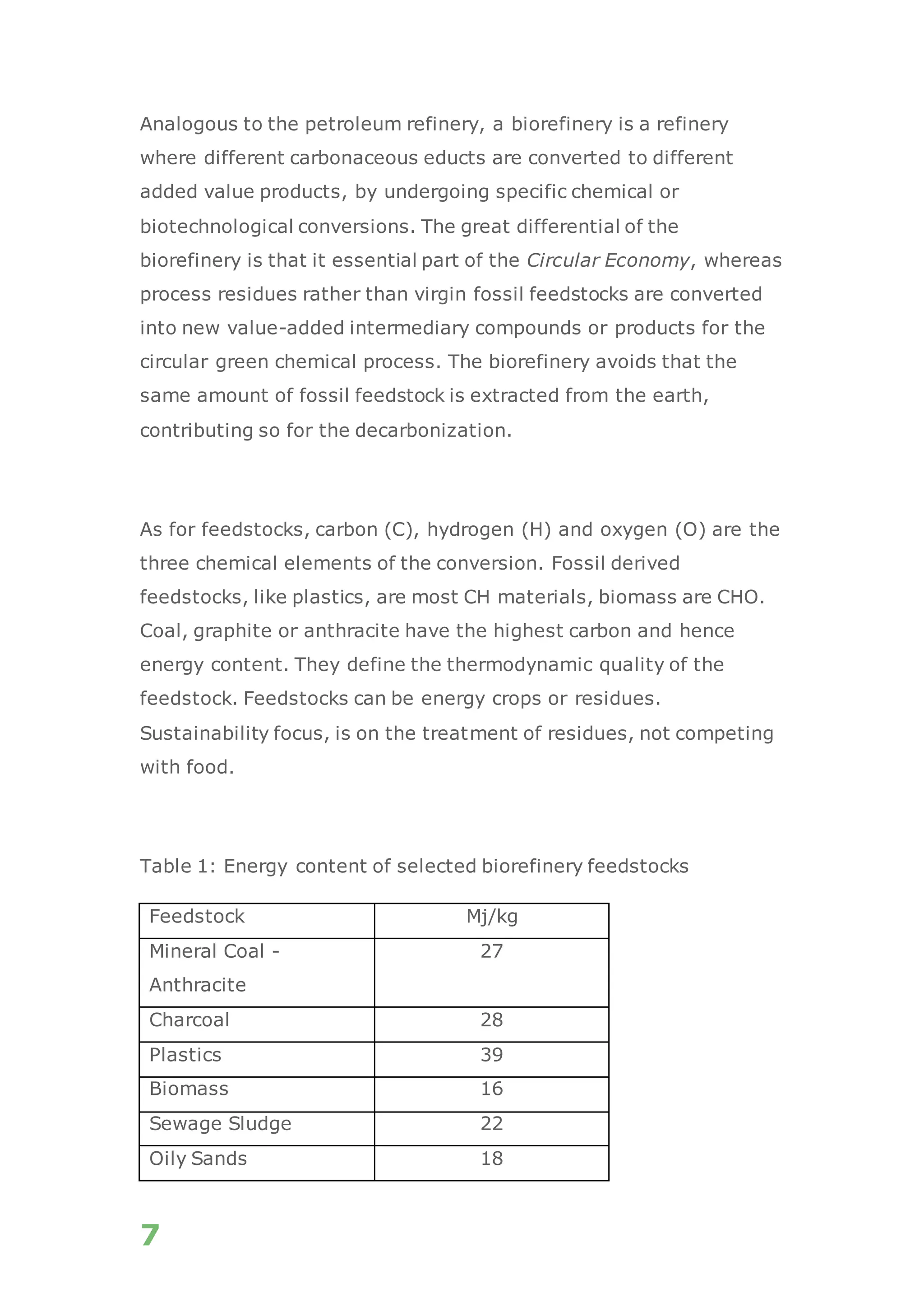
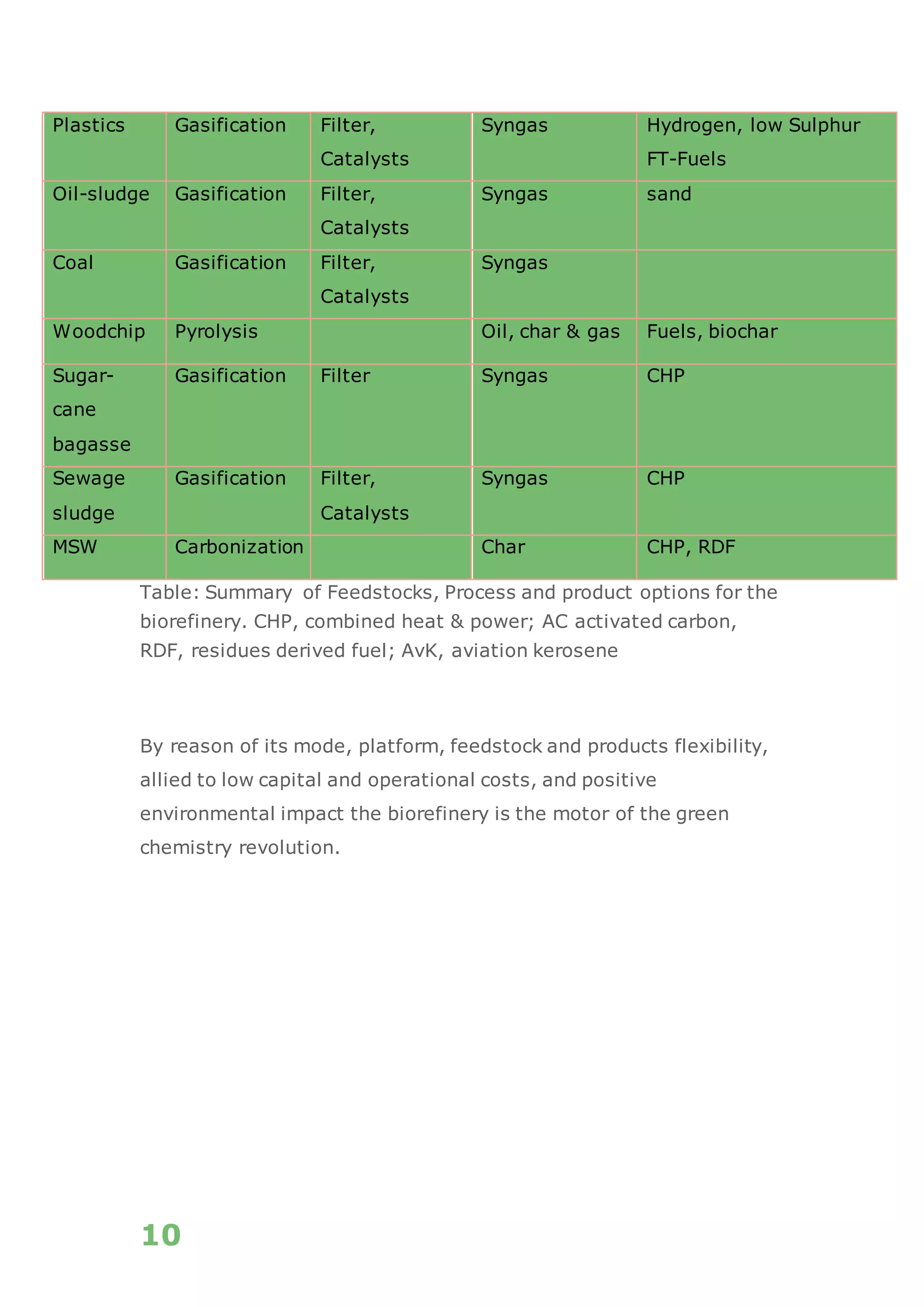
The white paper discusses the production and benefits of white hydrogen, a sustainable alternative derived from the gasification of non-recyclable plastics and biomass, which aligns with circular economy principles. It highlights the advanced gasification technology used by Recupera that ensures high purity of hydrogen while minimizing CO2 emissions. Additionally, it emphasizes hydrogen's critical role in the transition to a decarbonized economy and its various applications across sectors such as transportation, energy generation, and industry.