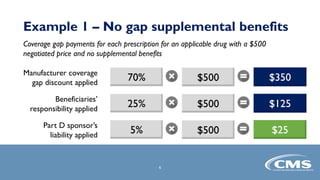
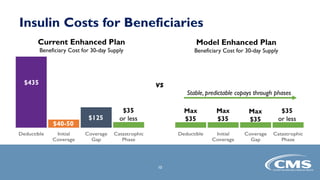
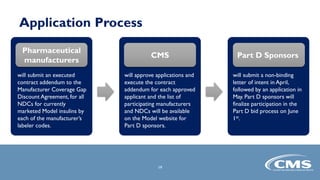
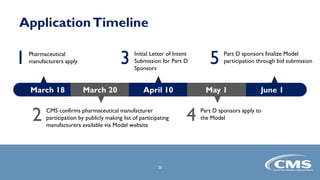
The document provides an overview of the Part D Senior Savings Model proposed by CMS Innovation Center. The model aims to lower out-of-pocket costs for insulin by establishing a stable $35 copay for eligible insulins through the deductible, initial coverage, and coverage gap phases of Part D plans. The model would be voluntary for manufacturers, Part D plans, and beneficiaries. It also outlines the application process and timelines for manufacturers and Part D plans to participate in the 2021 plan year.