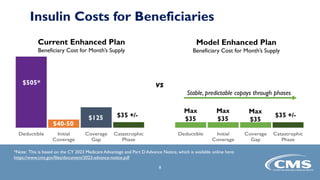
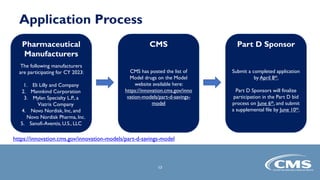
The Part D Senior Savings (PDSS) model for CY 2023 aims to provide beneficiaries with stable insulin costs capped at $35 for a one-month supply, addressing financial disincentives in Medicare's coverage gap. Participation is voluntary for manufacturers and Part D sponsors, with specific eligibility requirements outlined for enhanced benefit plans. Applications for participation are due by April 8, 2022, with details and resources provided for interested parties.