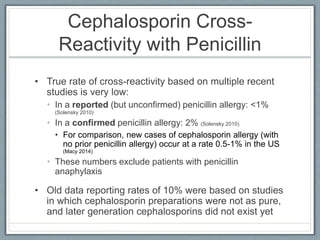
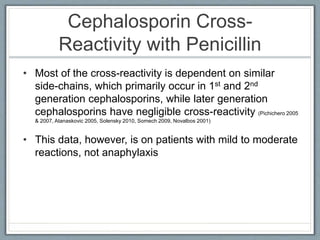
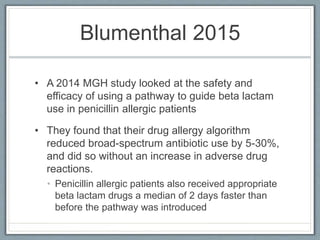
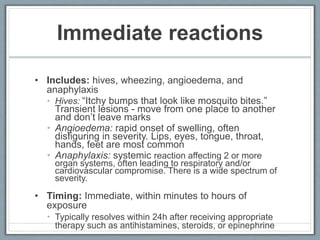
This document provides guidance on evaluating and managing patients who report a penicillin allergy. It begins by outlining the importance of accurately identifying penicillin allergies, as patients with reported allergies are more likely to receive broad-spectrum antibiotics and have worse outcomes. The document then describes a pathway for evaluating the type of reported penicillin reaction, identifying the appropriate antibiotic based on the reaction type, and safely administering test doses when needed to confirm allergy status. It emphasizes that most reported penicillin allergies are not actual allergies and that later-generation cephalosporins rarely cause reactions in truly penicillin-allergic patients. The pathway aims to improve antibiotic stewardship and allow more patients to safely