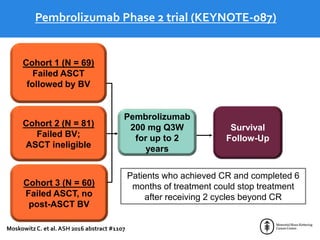
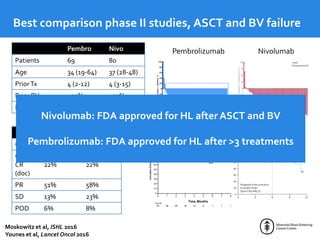
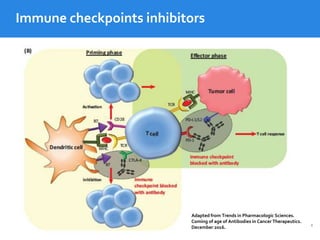
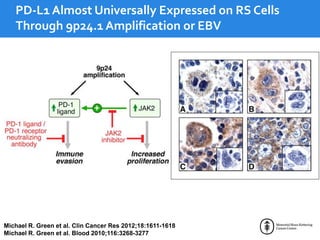
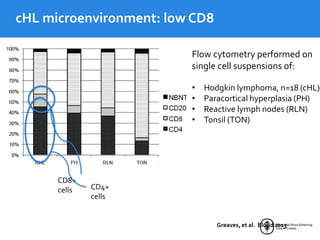
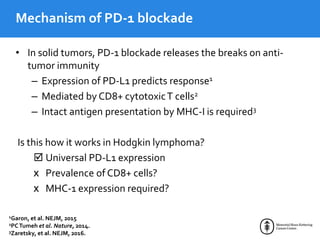
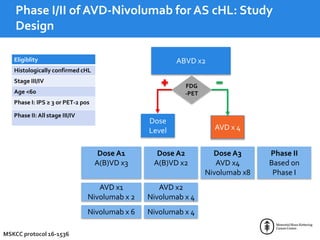
This document summarizes studies evaluating immune checkpoint inhibitors for the treatment of Hodgkin lymphoma. It discusses pivotal trials that led to FDA approval of nivolumab and pembrolizumab for relapsed/refractory HL after stem cell transplant and brentuximab vedotin. It also reviews mechanisms of action of PD-1 blockade in HL and efforts to combine checkpoint inhibitors with other agents or incorporate them into frontline treatment for high-risk patients.