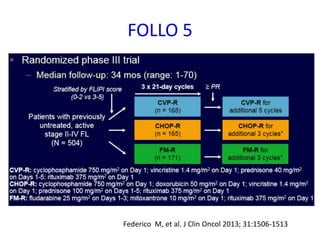
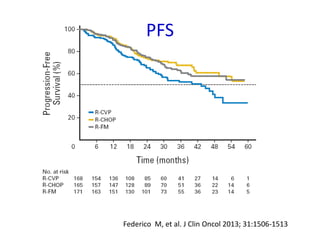
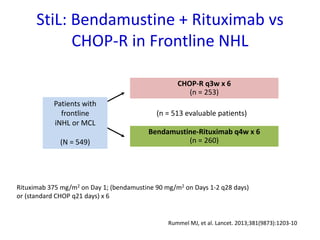
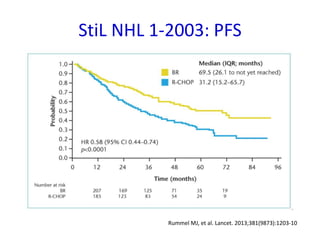
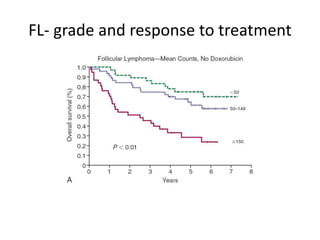
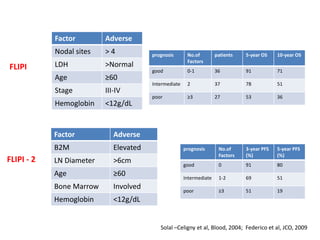
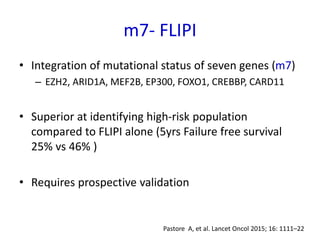
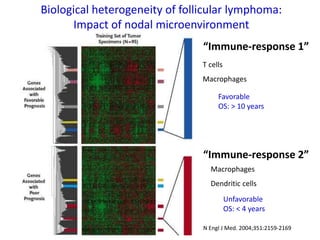
This document summarizes prognostic factors and treatment approaches for follicular lymphoma. Some key points:
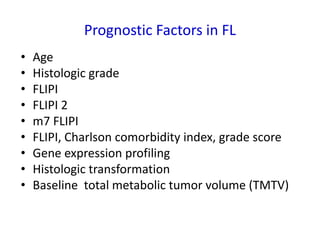
- Prognostic factors include age, histologic grade, FLIPI score, gene expression profiling, and metabolic tumor volume on PET scans.
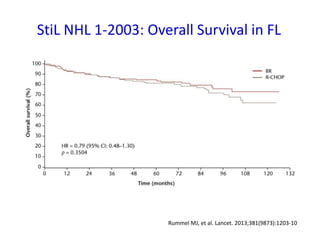
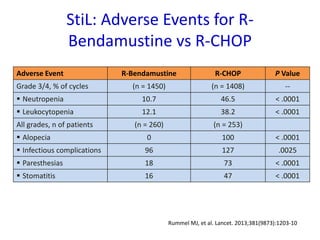
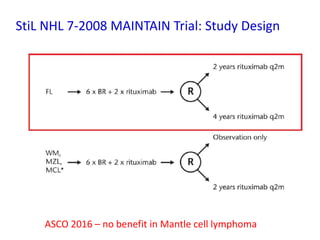
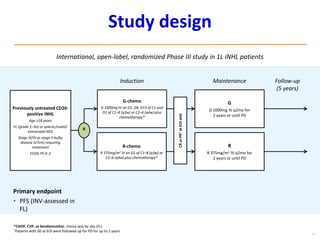
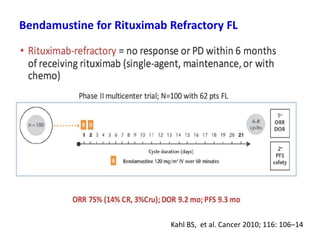
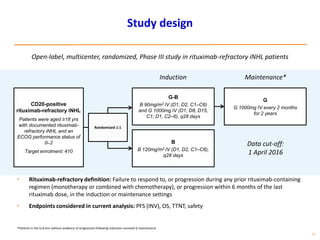
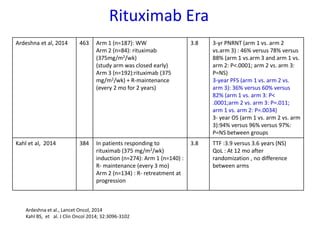
- First-line treatment for symptomatic advanced disease is usually rituximab plus chemotherapy such as bendamustine, followed by rituximab maintenance for 2 years.
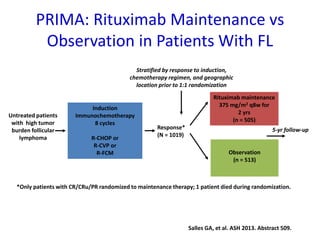
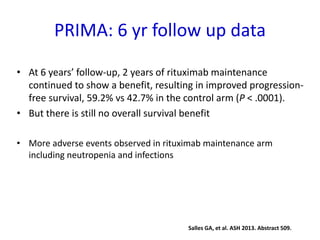
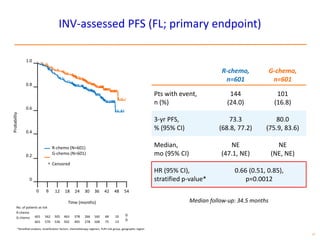
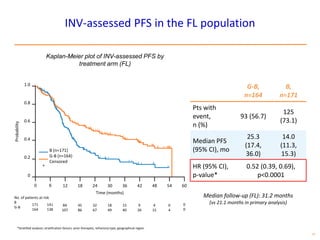
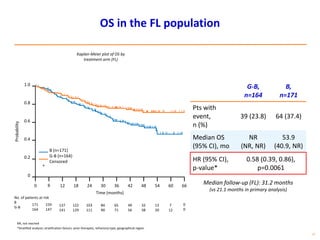
- The PRIMA trial showed improved progression-free survival with 2 years of rituximab maintenance compared to observation alone in patients achieving response to first-line treatment.
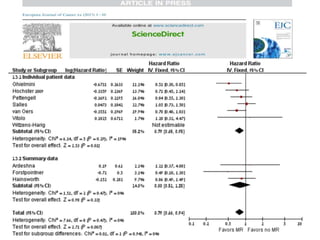
- The GALLIUM trial found improved progression-free survival for first-line treatment of follicular







![Wait and Watch
Randomized Trials: Low-Grade NHL
Pre –Rituximab Era
Trial Regimens FFS OS
Young 1988[1] ProMACE-MOPP + TNI vs watch and wait Yes No
Brice 1997[2] Prednimustine vs IF vs watch and wait No No
Ardeshna 2003[3] Chl vs watch and wait Yes No
1. Young RC, et al. Semin Hematol. 1988;25(2 suppl 2):11-16.
2. Brice P, et al. J Clin Oncol. 1997;15:1110-1117.
3. Ardeshna KM, et al. Lancet. 2003;362:516-522.](https://image.slidesharecdn.com/follicularlymphomaeafo2017-170510075306/85/V_Hematology_Forum_Dr_Pavithran-20-320.jpg)
![Addition of Rituximab to chemo
improves RR and survival
• CVP vs R-CVP[1]
• CHOP vs R-CHOP[2]
• MCP vs R-MCP[3]
1. Marcus R, et al. J Clin Oncol. 2008;26:4579-4586.
2. Hiddemann W, et al. Blood. 2005;106:3725-3732.
3. Herold M, et al. J Clin Oncol. 2007;25:1986-1992.](https://image.slidesharecdn.com/follicularlymphomaeafo2017-170510075306/85/V_Hematology_Forum_Dr_Pavithran-22-320.jpg)