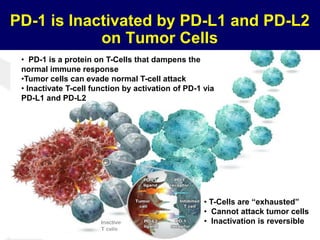
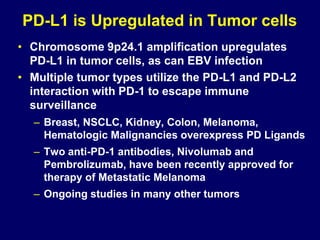
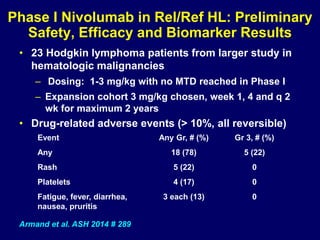
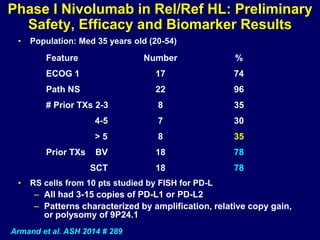
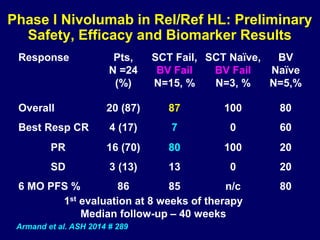
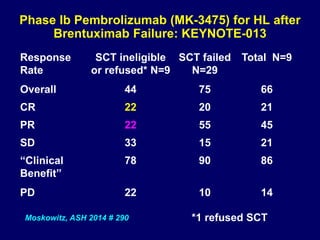
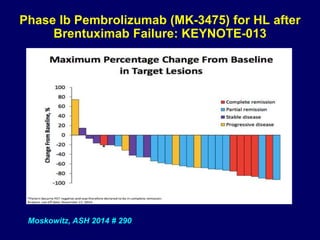
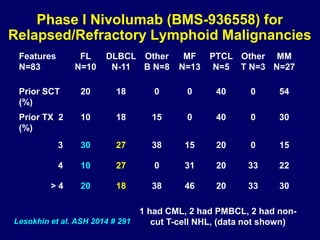
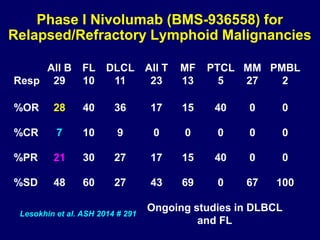
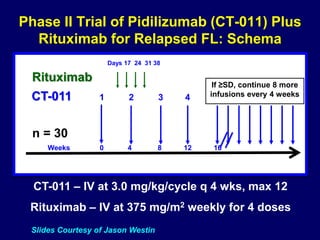
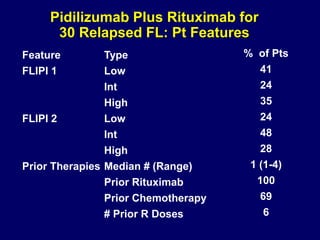
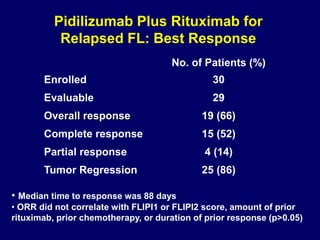
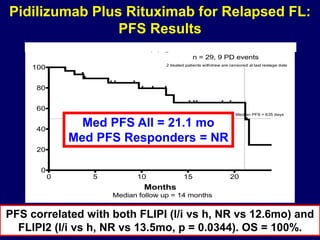
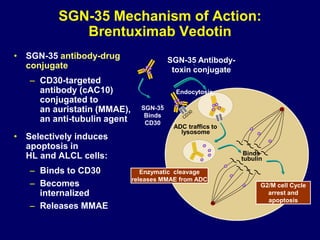
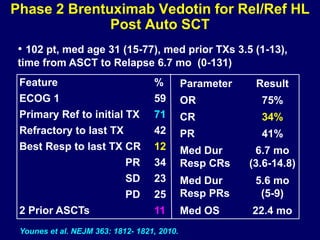
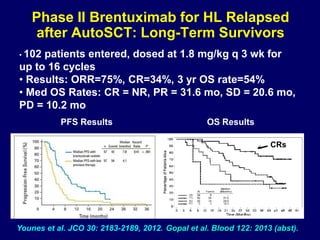
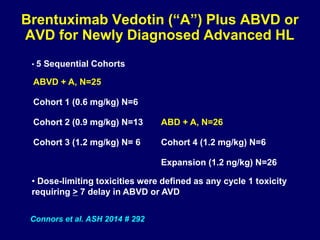
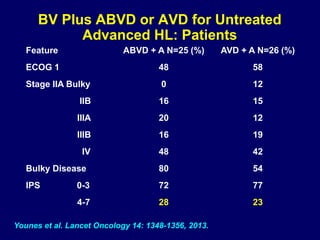
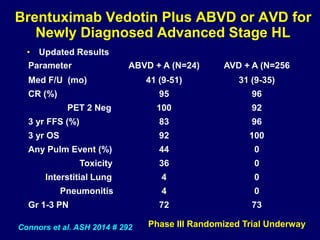
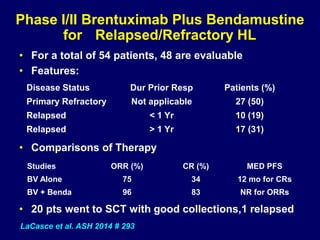
This document summarizes Dr. Frederick Hagemeister's presentation on anti-PD-1 and anti-CD30 antibodies for Hodgkin lymphoma from the 2014 American Society of Hematology annual meeting. It discusses preliminary results from phase 1 trials of nivolumab and pembrolizumab for relapsed/refractory HL, as well as trials combining brentuximab vedotin with chemotherapy for untreated advanced HL. Response rates to anti-PD-1 antibodies were high across studies. Combining brentuximab vedotin with ABVD/AVD showed promising efficacy in untreated patients with acceptable toxicity.