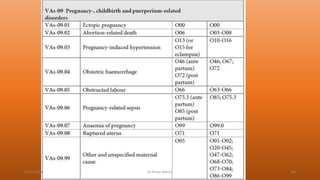
This document discusses verbal autopsy, including its definition, objective, need, uses, users, and historical background. Verbal autopsy is a method used to ascertain the cause of death based on an interview with caregivers after death. It provides important mortality data in places without robust death registration and certification systems. Standardization of verbal autopsy methods is needed to improve quality and comparability of results. The World Health Organization has developed standard verbal autopsy tools and methods to facilitate this.